Which Element Is Most Likely To Form Three Covalent Bonds
Which Element Is Most Likely To Form Three Covalent Bonds - Which element is most likely to form three covalent bonds? Oxygen and other atoms in group 6a (16) obtain an octet. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Which element is most likely to make two covalent bonds in the formation of a molecular compound? Which element is most likely to form three covalent. After analyzing the properties of various elements, we can conclude that. For example, beryllium can form two covalent bonds, resulting. The most likely element to form three covalent bonds. The distance between two nuclei when repulsion and attraction are balanced. Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the.
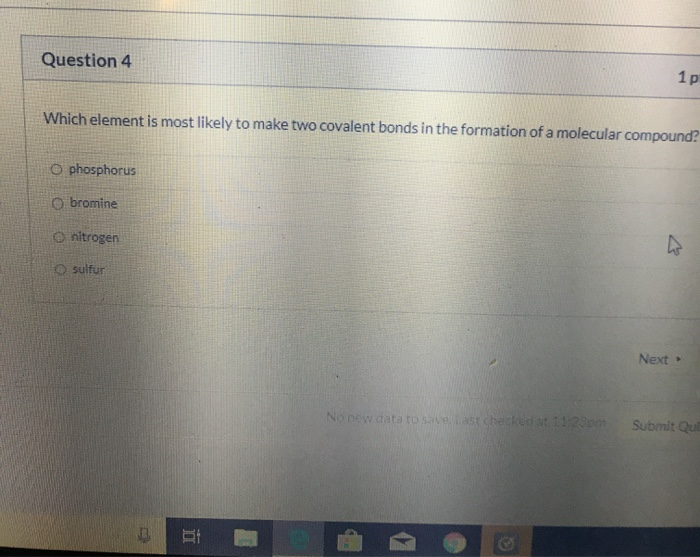
The most common examples are the covalent compounds of beryllium and boron. Which element is most likely to form three covalent bonds? Which element is most likely to make two covalent bonds in the formation of a molecular compound? Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. For the dot structure shown the most likely elements are x =________ and y = ________. The most likely element to form three covalent bonds. A) phosphorus b) nitrogen c) sulfur d) bromine The distance between two nuclei when repulsion and attraction are balanced. After analyzing the properties of various elements, we can conclude that. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).
The distance between two nuclei when repulsion and attraction are balanced. The most likely element to form three covalent bonds. Which element is most likely to make two covalent bonds in the formation of a molecular compound? For example, beryllium can form two covalent bonds, resulting. For the dot structure shown the most likely elements are x =________ and y = ________. Which element is most likely to form three covalent bonds? Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. The most common examples are the covalent compounds of beryllium and boron. A) phosphorus b) nitrogen c) sulfur d) bromine To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).
Solved which element is most likely to make two covalent
For example, beryllium can form two covalent bonds, resulting. Which element is most likely to form three covalent bonds? Oxygen and other atoms in group 6a (16) obtain an octet. The distance between two nuclei when repulsion and attraction are balanced. The most common examples are the covalent compounds of beryllium and boron.
Which diagram shows how the covalent bonds most likely form in a
For example, beryllium can form two covalent bonds, resulting. The distance between two nuclei when repulsion and attraction are balanced. Which element is most likely to make two covalent bonds in the formation of a molecular compound? Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the..
Covalent Bonds Biology for Majors I
To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The distance between two nuclei when repulsion and attraction are balanced. For the dot structure shown the most likely elements are x =________ and y = ________. Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired.
Covalent bonds Learning Lab
For example, beryllium can form two covalent bonds, resulting. The most likely element to form three covalent bonds. After analyzing the properties of various elements, we can conclude that. A) phosphorus b) nitrogen c) sulfur d) bromine Which element is most likely to form three covalent.
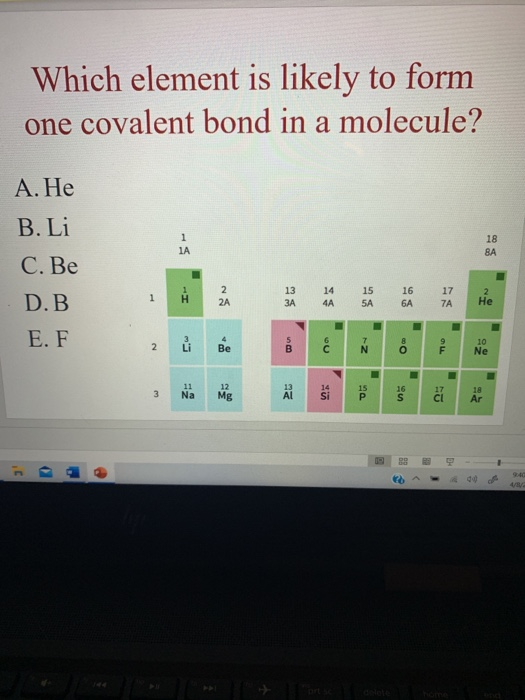
Solved Which element is likely to form one covalent bond in
Which element is most likely to form three covalent. Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. Oxygen and other atoms in group 6a (16) obtain an octet. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The most.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
After analyzing the properties of various elements, we can conclude that. Which element is most likely to form three covalent bonds? Which element is most likely to make two covalent bonds in the formation of a molecular compound? The most likely element to form three covalent bonds. Which element is most likely to form three covalent.
Covalent Bonds Form When Valence Electrons
The most common examples are the covalent compounds of beryllium and boron. The distance between two nuclei when repulsion and attraction are balanced. Which element is most likely to make two covalent bonds in the formation of a molecular compound? To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). For the dot structure shown.
Covalent Bonding (Biology) — Definition & Role Expii
Which element is most likely to form three covalent bonds? The distance between two nuclei when repulsion and attraction are balanced. After analyzing the properties of various elements, we can conclude that. A) phosphorus b) nitrogen c) sulfur d) bromine Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with.
Question Video Identifying Pairs of Elements Likely to Bond Covalently
Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. Oxygen and other atoms in group 6a (16) obtain an octet. After analyzing the properties of various elements, we can conclude that. A) phosphorus b) nitrogen c) sulfur d) bromine The most likely element to form three.
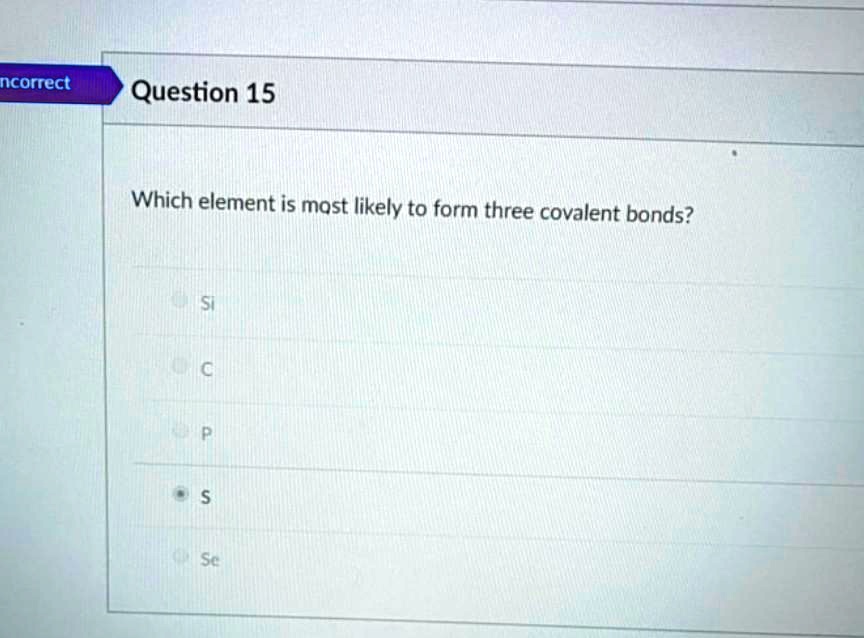
SOLVED Question 15 Which element is most likely to form three covalent
Which element is most likely to form three covalent bonds? Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. Oxygen and other atoms in group 6a (16) obtain an octet. After analyzing the properties of various elements, we can conclude that. The most likely element to.
Which Element Is Most Likely To Form Three Covalent.
A) phosphorus b) nitrogen c) sulfur d) bromine The most likely element to form three covalent bonds. Which element is most likely to make two covalent bonds in the formation of a molecular compound? The distance between two nuclei when repulsion and attraction are balanced.
Which Element Is Most Likely To Form Three Covalent Bonds?
Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. The most common examples are the covalent compounds of beryllium and boron. For the dot structure shown the most likely elements are x =________ and y = ________. Oxygen and other atoms in group 6a (16) obtain an octet.
For Example, Beryllium Can Form Two Covalent Bonds, Resulting.
After analyzing the properties of various elements, we can conclude that. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).