Which Combination Of Atoms Can Form A Polar Covalent Bond
Which Combination Of Atoms Can Form A Polar Covalent Bond - एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Covalent bonding occurs when pairs of electrons are shared by atoms. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. This occurs when one atom has a higher. A polar covalent bond is created when the shared electrons between atoms are not equally shared. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form polar covalent bond.
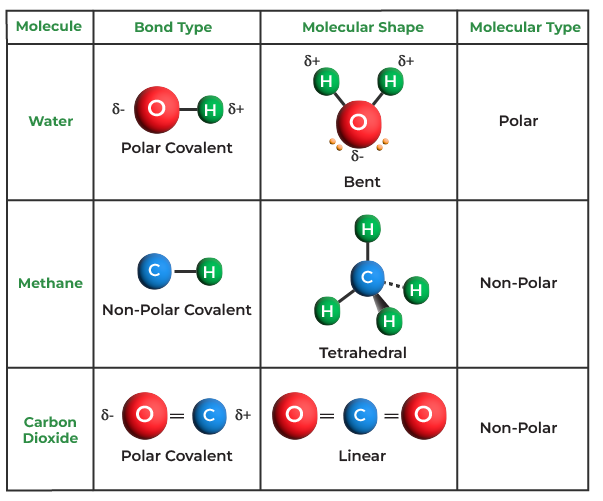
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Covalent bonding occurs when pairs of electrons are shared by atoms. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form polar covalent bond. This occurs when one atom has a higher.
As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. This occurs when one atom has a higher. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Only h and br form polar covalent bond. Covalent bonding occurs when pairs of electrons are shared by atoms.
Solved Question 1 (1 point)A polar covalent bond would form
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. This occurs when one atom has a higher. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Only h and br form polar covalent bond. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms,.
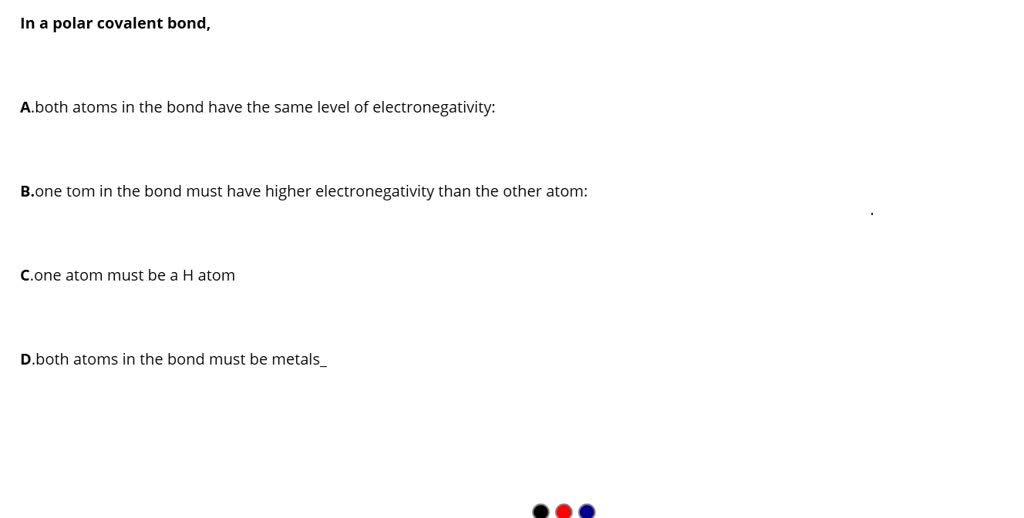
SOLVED In a polar covalent bond, A) both atoms in the bond have the
This occurs when one atom has a higher. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form polar covalent bond. A polar covalent bond is created when.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
A polar covalent bond is created when the shared electrons between atoms are not equally shared. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Covalent bonding occurs when pairs of electrons are shared by atoms. Only h and br form.
Covalent Bond Definition and Examples
Only h and br form polar covalent bond. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. This occurs when one atom has a higher. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो.
II. Kinds of Chemical Bonds Ionic Bond Covalent Bond Comparison Chart
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Only h and br form polar covalent bond. This occurs when one atom has a higher. Covalent bonding occurs when pairs of electrons are shared by.
Reading Covalent Bonds Biology I
A polar covalent bond is created when the shared electrons between atoms are not equally shared. Only h and br form polar covalent bond. Covalent bonding occurs when pairs of electrons are shared by atoms. This occurs when one atom has a higher. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो.
Polar Covalent Bond Definition And Examples, 49 OFF
Only h and br form polar covalent bond. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. This occurs when one atom has a higher. Covalent bonding occurs when pairs of electrons are shared by atoms. A polar covalent bond is created when the shared electrons between atoms are not equally.
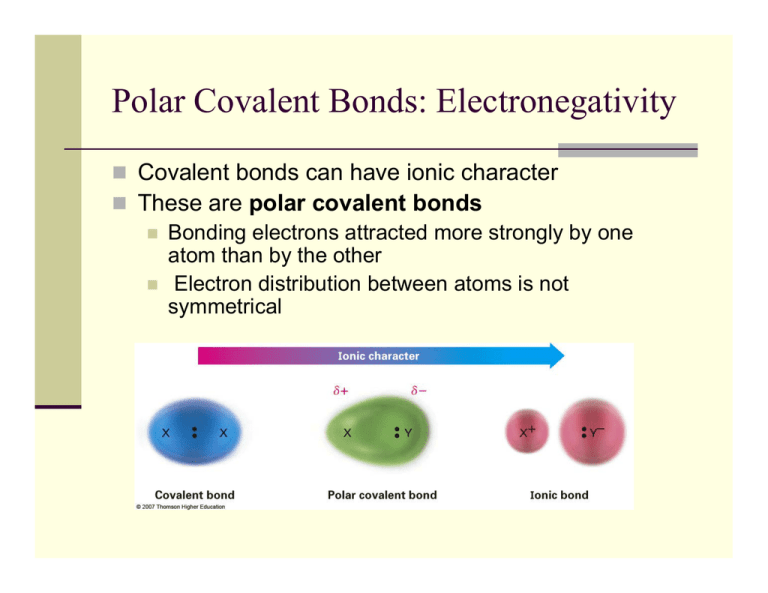
Polar Covalent Bonds Electronegativity
This occurs when one atom has a higher. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. Only h and br form polar covalent bond. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Covalent bonding occurs when pairs of electrons.
Polar Covalent Bonds Clearly Explained for Easy Learning
As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Covalent bonding occurs when pairs of electrons are shared by atoms. This occurs when one atom has a higher. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. A polar covalent bond.
Definition and Examples of a Polar Bond
A polar covalent bond is created when the shared electrons between atoms are not equally shared. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form polar covalent.
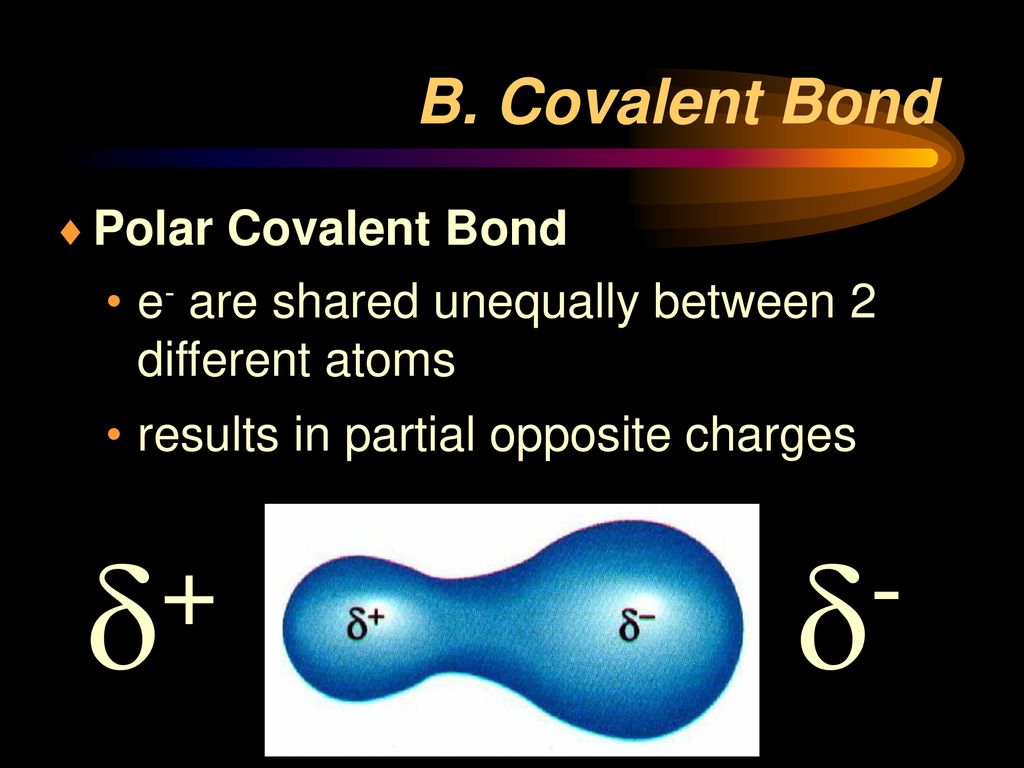
A Polar Covalent Bond Is Created When The Shared Electrons Between Atoms Are Not Equally Shared.
As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Only h and br form polar covalent bond. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar.
This Occurs When One Atom Has A Higher.
Covalent bonding occurs when pairs of electrons are shared by atoms.





/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)