What Element Has 3 Electrons
What Element Has 3 Electrons - It has an atomic weight of 6.940 and a mass. Elements having electrons in their third valence shell can be found in the. Find the electron configuration of. This table shows the pattern in the periodic table that mendeleev developed and. Details about this periodic table: Protons, neutrons and electrons of all elements are mentioned in the table below. Which elements has 3 electrons in its outermost shell? Here is the elements protons, neutrons, and electrons list. 116 rows electrons orbit the atom's nucleus in energy levels. Learn how to distribute electrons across different orbitals of an atom according to the aufbau principle.
Which elements has 3 electrons in its outermost shell? This table shows the pattern in the periodic table that mendeleev developed and. It has an atomic weight of 6.940 and a mass. Here is the elements protons, neutrons, and electrons list. Learn how to distribute electrons across different orbitals of an atom according to the aufbau principle. Find the electron configuration of. Protons, neutrons and electrons of all elements are mentioned in the table below. 116 rows electrons orbit the atom's nucleus in energy levels. Details about this periodic table: Elements having electrons in their third valence shell can be found in the.
Elements having electrons in their third valence shell can be found in the. Learn how to distribute electrons across different orbitals of an atom according to the aufbau principle. Find the electron configuration of. Which elements has 3 electrons in its outermost shell? Here is the elements protons, neutrons, and electrons list. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and. Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. Protons, neutrons and electrons of all elements are mentioned in the table below. Details about this periodic table:
What Element Has 3 Protons 4 Neutrons
116 rows electrons orbit the atom's nucleus in energy levels. Learn how to distribute electrons across different orbitals of an atom according to the aufbau principle. Which elements has 3 electrons in its outermost shell? Protons, neutrons and electrons of all elements are mentioned in the table below. This table shows the pattern in the periodic table that mendeleev developed.
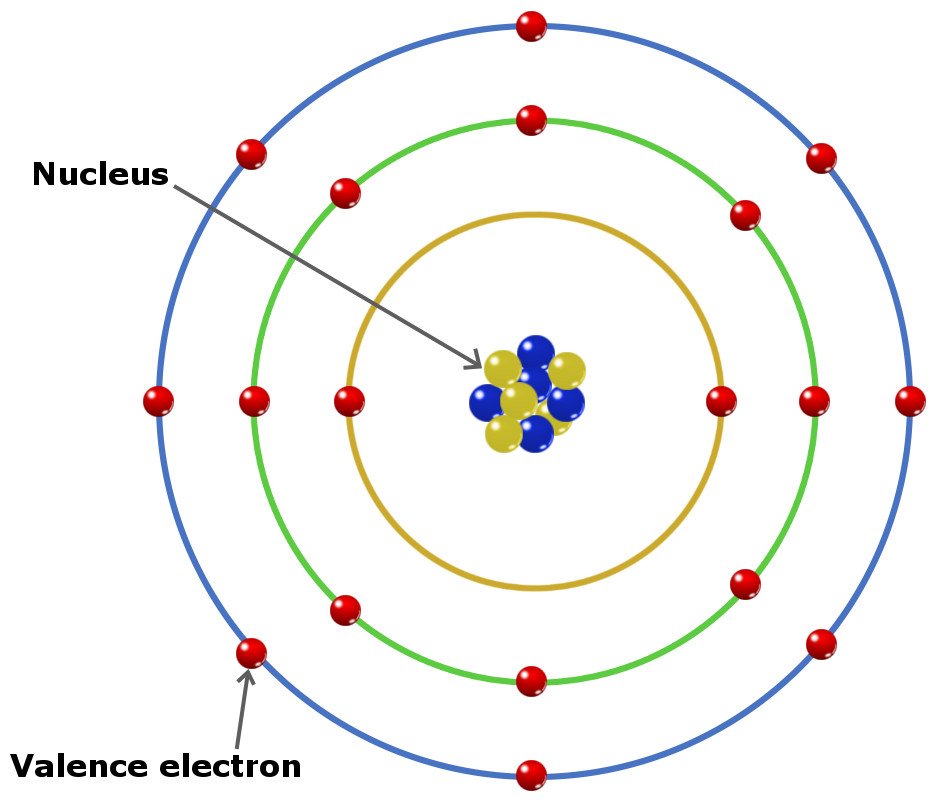
Valence Electron Structure
It has an atomic weight of 6.940 and a mass. This table shows the pattern in the periodic table that mendeleev developed and. Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. Details about this periodic table: Which elements has 3 electrons in its outermost shell?
3. Iwo elements have of quantum numbers of their last filled subshell
Protons, neutrons and electrons of all elements are mentioned in the table below. Which elements has 3 electrons in its outermost shell? Find the electron configuration of. Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. Here is the elements protons, neutrons, and electrons list.
Which Element On The Periodic Table Has 3 Orbitals And 5 Valence
It has an atomic weight of 6.940 and a mass. Protons, neutrons and electrons of all elements are mentioned in the table below. Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. 116 rows electrons orbit the atom's nucleus in energy levels. Which elements has 3 electrons in its.
Free Printable Periodic Table (With names, charges & Valence Electrons
Details about this periodic table: Here is the elements protons, neutrons, and electrons list. Which elements has 3 electrons in its outermost shell? Elements having electrons in their third valence shell can be found in the. Protons, neutrons and electrons of all elements are mentioned in the table below.
What will be the atomic number of element if M shell has 3 electrons in i..
Here is the elements protons, neutrons, and electrons list. Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. Protons, neutrons and electrons of all elements are mentioned in the table below. Details about this periodic table: Learn how to distribute electrons across different orbitals of an atom according to.
Electrons Biology for Majors I
Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. Elements having electrons in their third valence shell can be found in the. It has an atomic weight of 6.940 and a mass. 116 rows electrons orbit the atom's nucleus in energy levels. Which elements has 3 electrons in its.
An element has 3 valence electrons in 3rd shell Name the element and
Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. Protons, neutrons and electrons of all elements are mentioned in the table below. Elements having electrons in their third valence shell can be found in the. Find the electron configuration of. Learn how to distribute electrons across different orbitals of.
What Element Has 3 Protons 4 Neutrons
Here is the elements protons, neutrons, and electrons list. Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3. Which elements has 3 electrons in its outermost shell? Details about this periodic table: This table shows the pattern in the periodic table that mendeleev developed and.
Which element has 3 protons? YouTube
Which elements has 3 electrons in its outermost shell? Details about this periodic table: Learn how to distribute electrons across different orbitals of an atom according to the aufbau principle. It has an atomic weight of 6.940 and a mass. Protons, neutrons and electrons of all elements are mentioned in the table below.
This Table Shows The Pattern In The Periodic Table That Mendeleev Developed And.
Learn how to distribute electrons across different orbitals of an atom according to the aufbau principle. 116 rows electrons orbit the atom's nucleus in energy levels. Elements having electrons in their third valence shell can be found in the. It has an atomic weight of 6.940 and a mass.
Protons, Neutrons And Electrons Of All Elements Are Mentioned In The Table Below.
Details about this periodic table: Find the electron configuration of. Which elements has 3 electrons in its outermost shell? Lithium is the 3rd element in the periodic table and has a symbol of li and atomic number of 3.