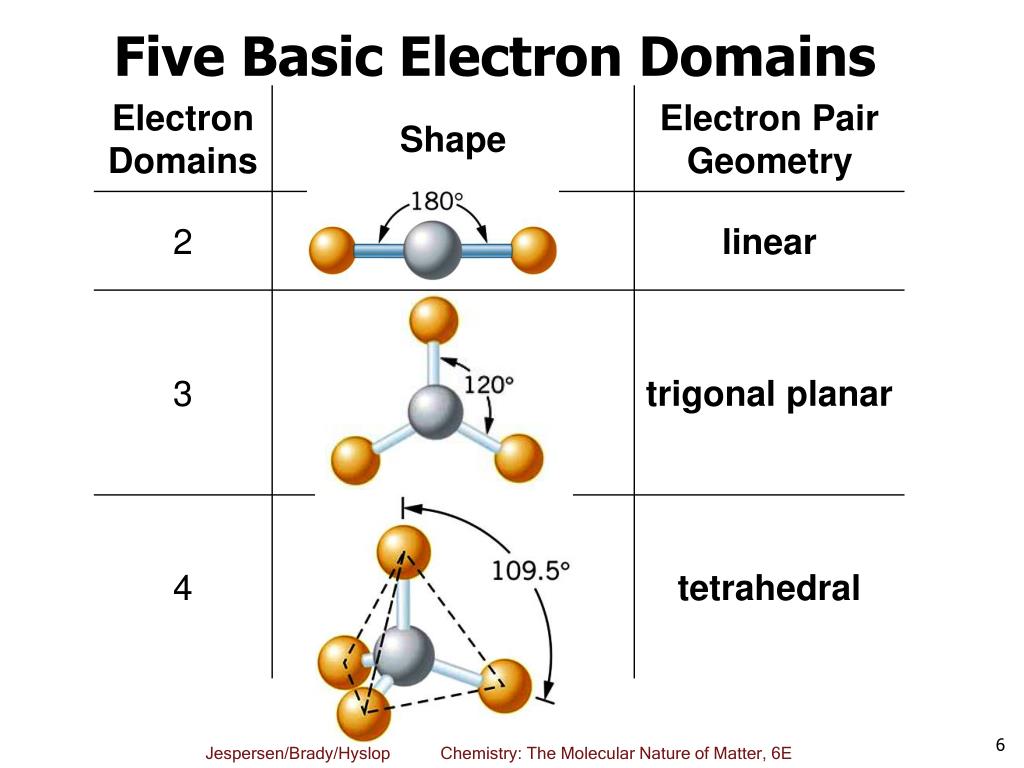
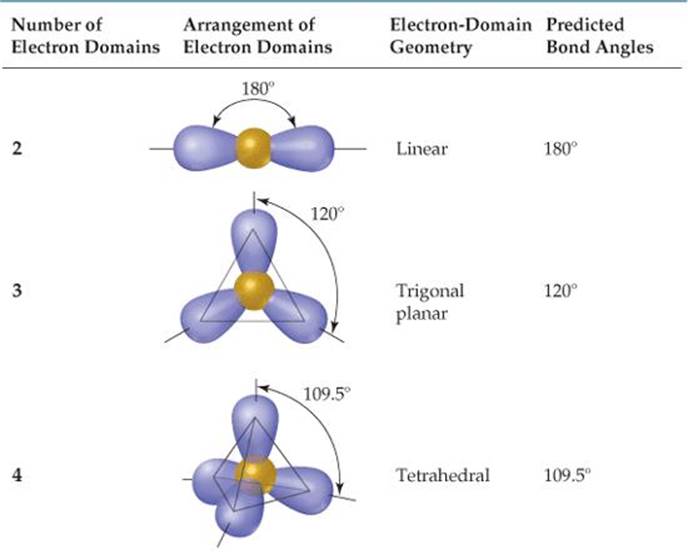
What Are Electron Domains
What Are Electron Domains - Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. Electron domains are the units of. An electron domain is the region of space around an atom where one or more electrons are found. A lone pair, single, double, or triple. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. It is a fundamental concept in the study of atomic.
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. An electron domain is the region of space around an atom where one or more electrons are found. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. Electron domains are the units of. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. It is a fundamental concept in the study of atomic. A lone pair, single, double, or triple.
Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. It is a fundamental concept in the study of atomic. An electron domain is the region of space around an atom where one or more electrons are found. Electron domains are the units of. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. A lone pair, single, double, or triple.
Electron Domain Geometry Chart
A lone pair, single, double, or triple. Electron domains are the units of. An electron domain is the region of space around an atom where one or more electrons are found. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. It is a fundamental concept in the study of atomic.
THE VSEPR MODEL MOLECULAR GEOMETRY AND BONDING THEORIES CHEMISTRY
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. An electron domain is the region of space around an atom where one or more electrons are found. A lone pair, single, double, or triple. Learn how to use the electron domain theory to predict the shapes and polarities of molecules.
PPT Chapter 10 Theories of Bonding and Structure PowerPoint
It is a fundamental concept in the study of atomic. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. An electron domain is the region of space around an atom where one or more electrons are found. Learn how vsepr explains the shape and structure of molecules based on the.
Ch 9 Electron Domains & Molecular Geometries 1 YouTube
An electron domain is the region of space around an atom where one or more electrons are found. It is a fundamental concept in the study of atomic. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Learn how vsepr explains the shape and structure of molecules based on the.
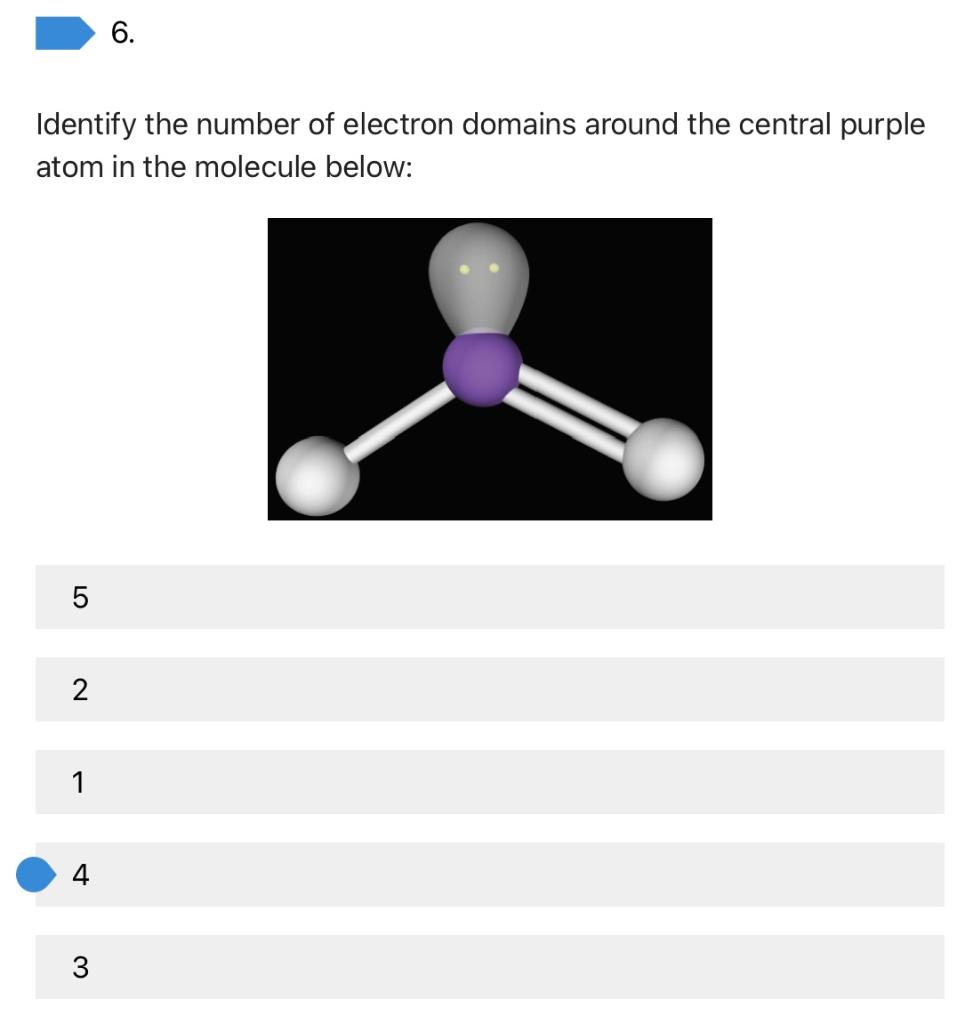
Solved 6. Identify the number of electron domains around the
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. Learn how vsepr explains the shape and structure of molecules based on the repulsion of.
Electron Domain Definition and VSEPR Theory
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. A lone pair, single, double, or triple. Electron domains are the units of. Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. Learn how vsepr explains the shape and structure of.
Electron Domain and Molecular Geometry Diagram Quizlet
Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or. A lone pair, single, double, or triple. An electron domain is the region of space around an atom where one or more electrons are found. Learn how to use the electron domain theory to.
PPT Chapter 10 Theories of Bonding and Structure PowerPoint
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. A lone pair, single, double, or triple. Electron domains are the units of. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. Electron domains refer to regions around a central atom in a molecule.
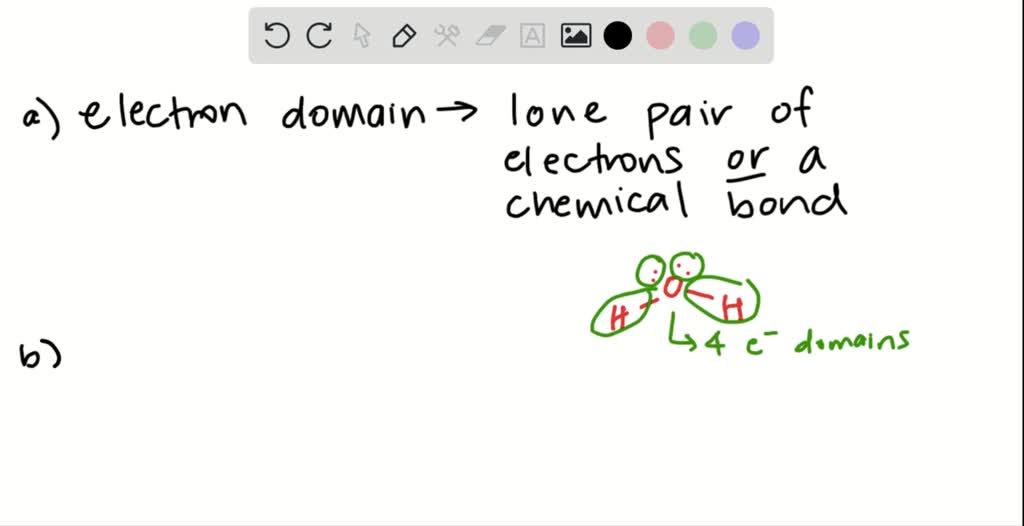
SOLVED(a) What is meant by the term electron domadit? (b) Explain in
An electron domain is the region of space around an atom where one or more electrons are found. Electron domains are the units of. Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. A lone pair, single, double, or triple. It is a fundamental concept in the study of atomic.
How to Determine the Number of Electron Domains and the Molecular
Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. An electron domain is the region of space around an atom where one or more electrons are found. A lone pair, single, double, or triple. There are two molecular geometries that can come out of three electron domains, trigonal planar (no.
A Lone Pair, Single, Double, Or Triple.
Learn how to use the electron domain theory to predict the shapes and polarities of molecules with covalent bonds. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and. Electron domains are the units of. Electron domains refer to regions around a central atom in a molecule where electrons are likely to be found, such as bonding pairs, lone pairs, or.
An Electron Domain Is The Region Of Space Around An Atom Where One Or More Electrons Are Found.
Learn how vsepr explains the shape and structure of molecules based on the repulsion of electrons. It is a fundamental concept in the study of atomic.

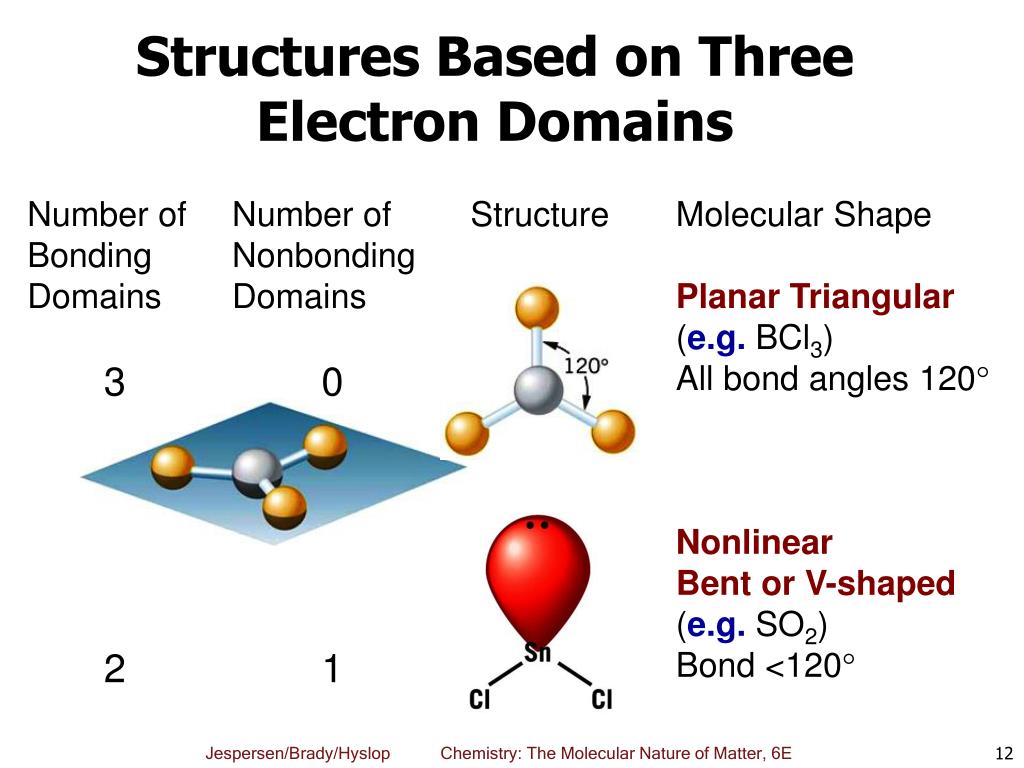

/carbon-dioxide-molecule-545861181-5934474d3df78c08ab2ba23f.jpg)