Relationship Between Heat And Temperature
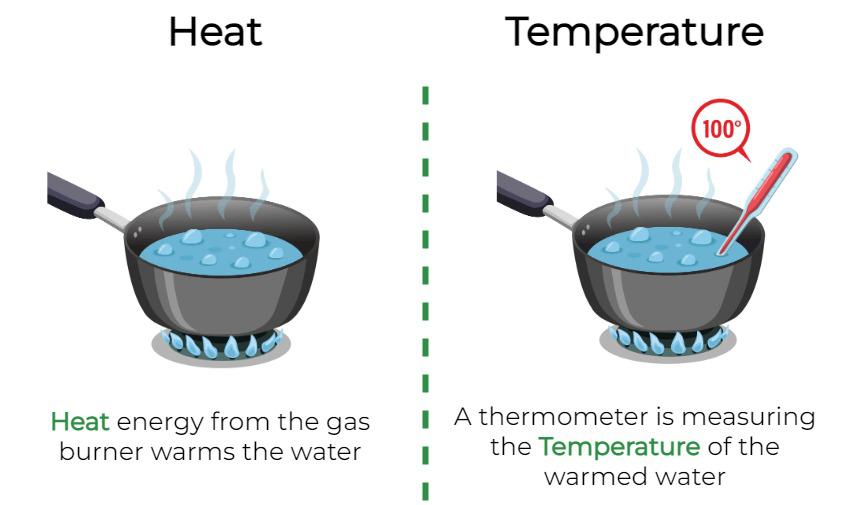
Relationship Between Heat And Temperature - Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. Heat is the energy that flows as a result of a difference in temperature. We use the symbol q for heat. Discover the fundamental concepts of heat and temperature with our straightforward guide. Heat, like all forms of energy,. Heat is the flow of energy from one object to another. This flow of energy is caused by a difference in temperature. Learn about thermal energy and its relationship to.
Heat is the energy that flows as a result of a difference in temperature. Learn about thermal energy and its relationship to. This flow of energy is caused by a difference in temperature. Heat is the flow of energy from one object to another. We use the symbol q for heat. Discover the fundamental concepts of heat and temperature with our straightforward guide. Heat, like all forms of energy,. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average.
Heat, like all forms of energy,. Discover the fundamental concepts of heat and temperature with our straightforward guide. This flow of energy is caused by a difference in temperature. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. Heat is the flow of energy from one object to another. We use the symbol q for heat. Learn about thermal energy and its relationship to. Heat is the energy that flows as a result of a difference in temperature.
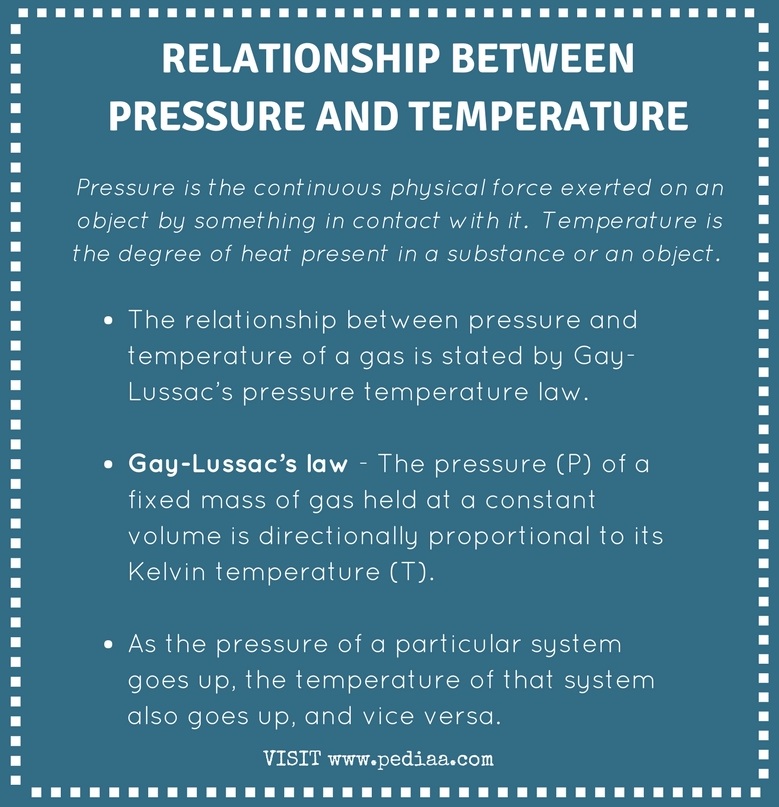
Relationship Between Pressure and Temperature
This flow of energy is caused by a difference in temperature. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. Heat is the flow of energy from one object to another. Heat, like all forms of energy,. Learn about thermal energy and its relationship to.
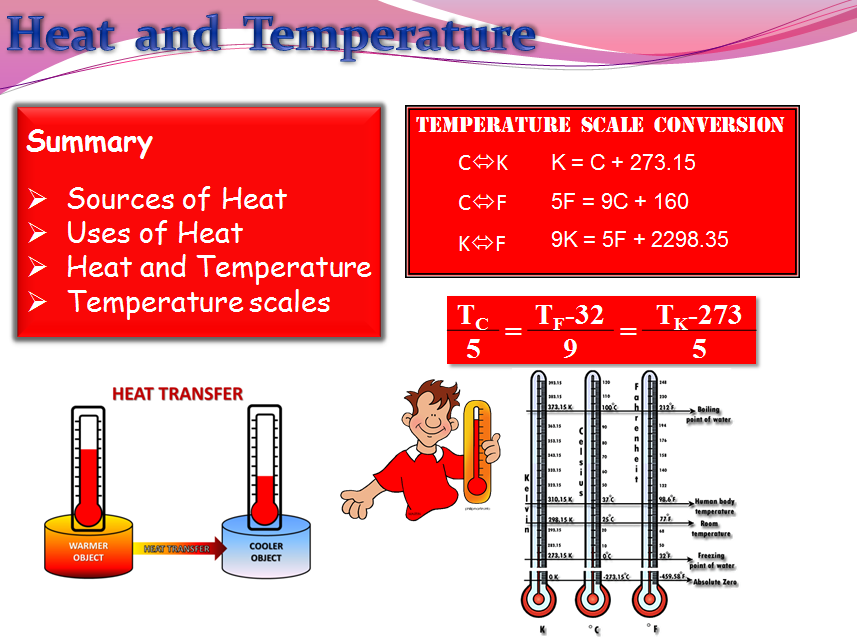
Energy & Transformations ppt download
We use the symbol q for heat. Heat is the flow of energy from one object to another. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. Heat is the energy that flows as a result of a difference in temperature. Learn about thermal energy and.
Climate Science Investigations South Florida Energy The Driver of
We use the symbol q for heat. Heat is the energy that flows as a result of a difference in temperature. Heat is the flow of energy from one object to another. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. Learn about thermal energy and.
Relationship between specific heat capacity and temperature from the
Discover the fundamental concepts of heat and temperature with our straightforward guide. This flow of energy is caused by a difference in temperature. Heat is the energy that flows as a result of a difference in temperature. Heat, like all forms of energy,. Learn about thermal energy and its relationship to.
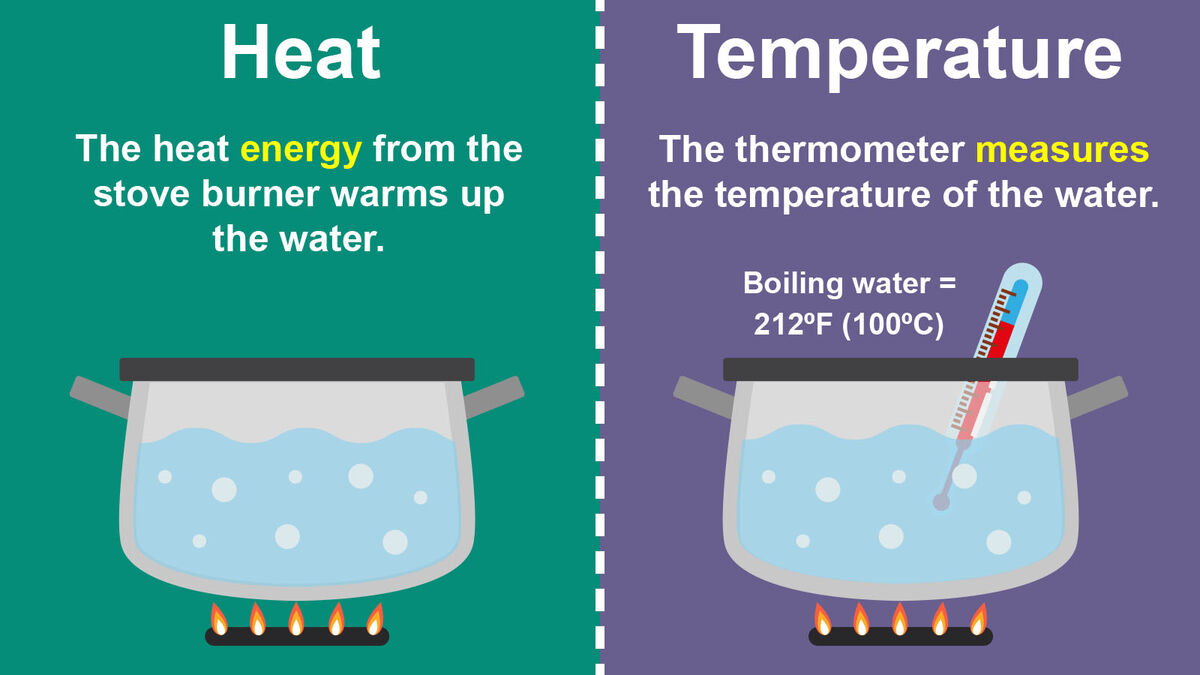
Heat Vs Temperature Examples
Learn about thermal energy and its relationship to. Heat, like all forms of energy,. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. Heat is the energy that flows as a result of a difference in temperature. We use the symbol q for heat.
What's the Difference between Heat and Temperature? YouTube
Heat is the energy that flows as a result of a difference in temperature. This flow of energy is caused by a difference in temperature. Learn about thermal energy and its relationship to. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. We use the symbol.
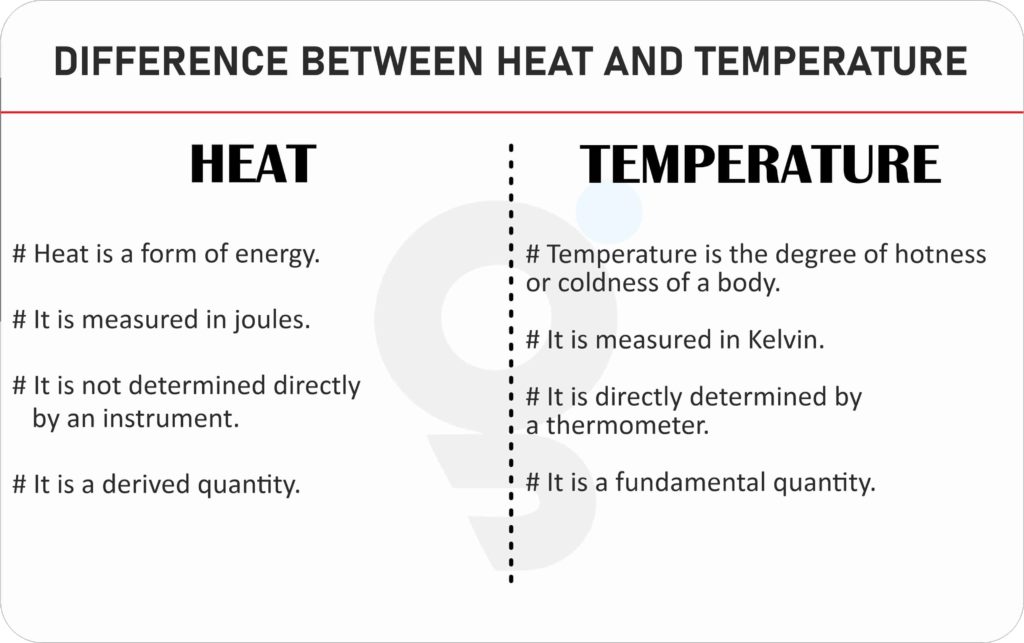
8 Remarkable Difference between Heat and Temperature Core Differences
Learn about thermal energy and its relationship to. Heat is the flow of energy from one object to another. This flow of energy is caused by a difference in temperature. We use the symbol q for heat. Heat is the energy that flows as a result of a difference in temperature.
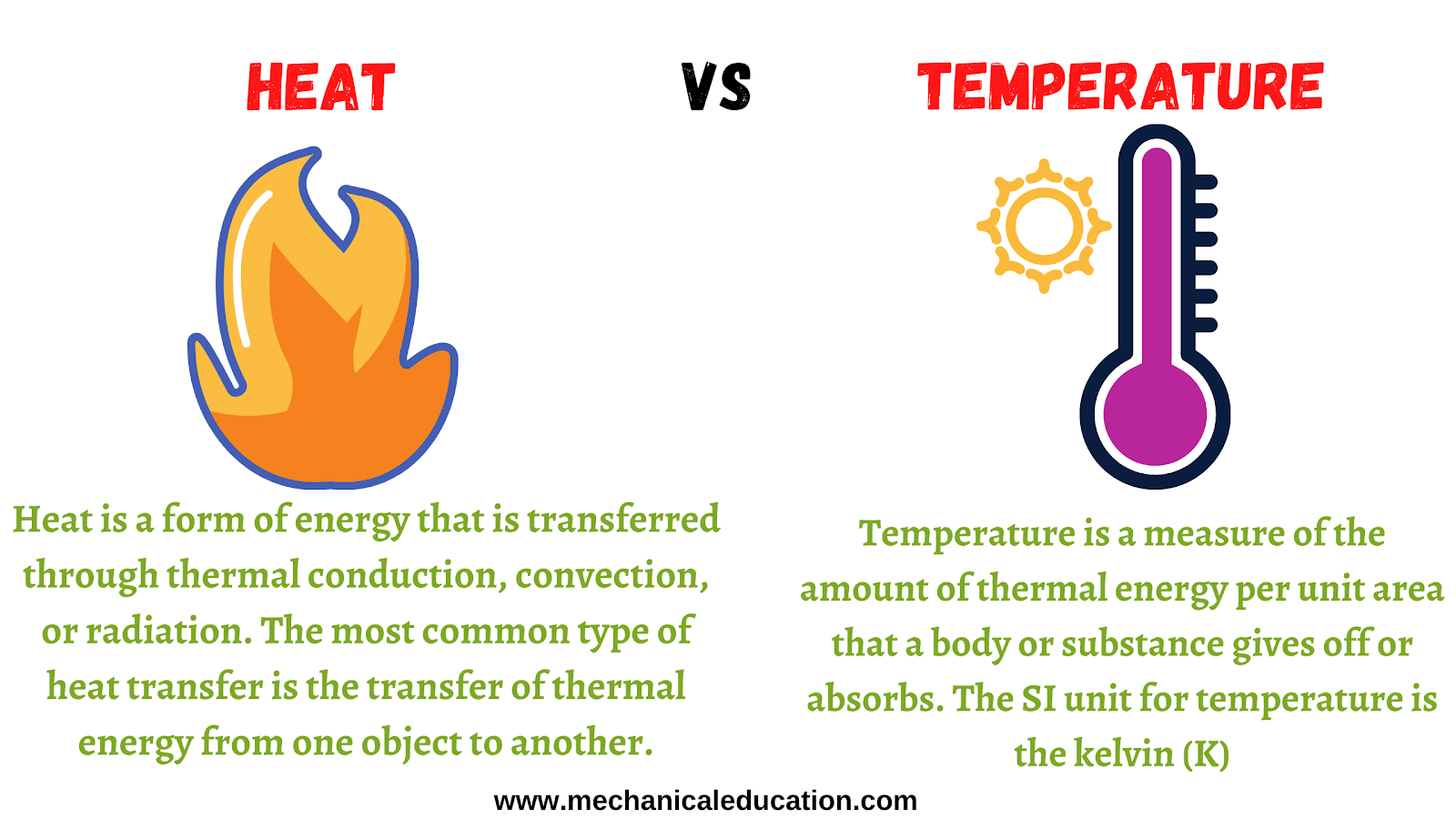
Difference between Heat and Temperature Mechanical Education
Discover the fundamental concepts of heat and temperature with our straightforward guide. Heat, like all forms of energy,. We use the symbol q for heat. Heat is the flow of energy from one object to another. Heat is the energy that flows as a result of a difference in temperature.
What is Difference between Heat and Temperature?
Heat is the energy that flows as a result of a difference in temperature. Heat is the flow of energy from one object to another. We use the symbol q for heat. Learn about thermal energy and its relationship to. Discover the fundamental concepts of heat and temperature with our straightforward guide.
Difference Between Heat and Temperature in Simple Terms YourDictionary
Heat, like all forms of energy,. Discover the fundamental concepts of heat and temperature with our straightforward guide. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. This flow of energy is caused by a difference in temperature. Heat is the energy that flows as a.
We Use The Symbol Q For Heat.
Discover the fundamental concepts of heat and temperature with our straightforward guide. Heat is the flow of energy from one object to another. Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. Learn about thermal energy and its relationship to.
Heat, Like All Forms Of Energy,.
Heat is the energy that flows as a result of a difference in temperature. This flow of energy is caused by a difference in temperature.