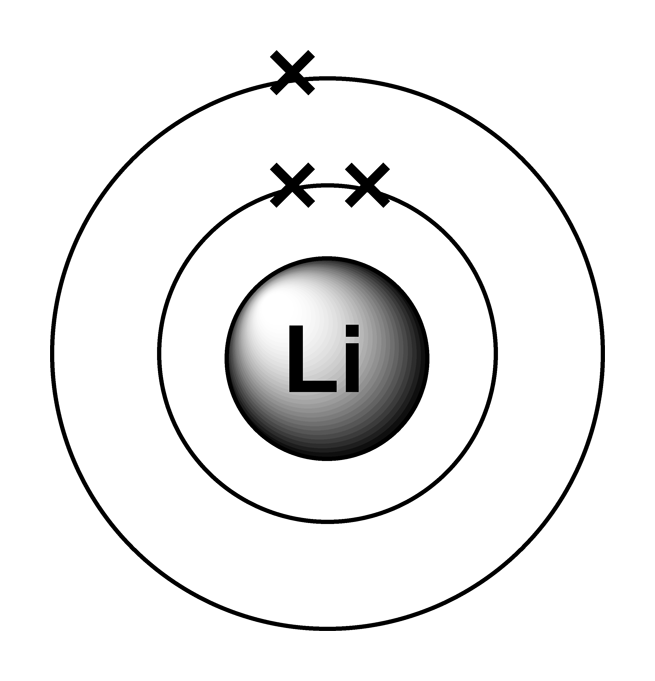
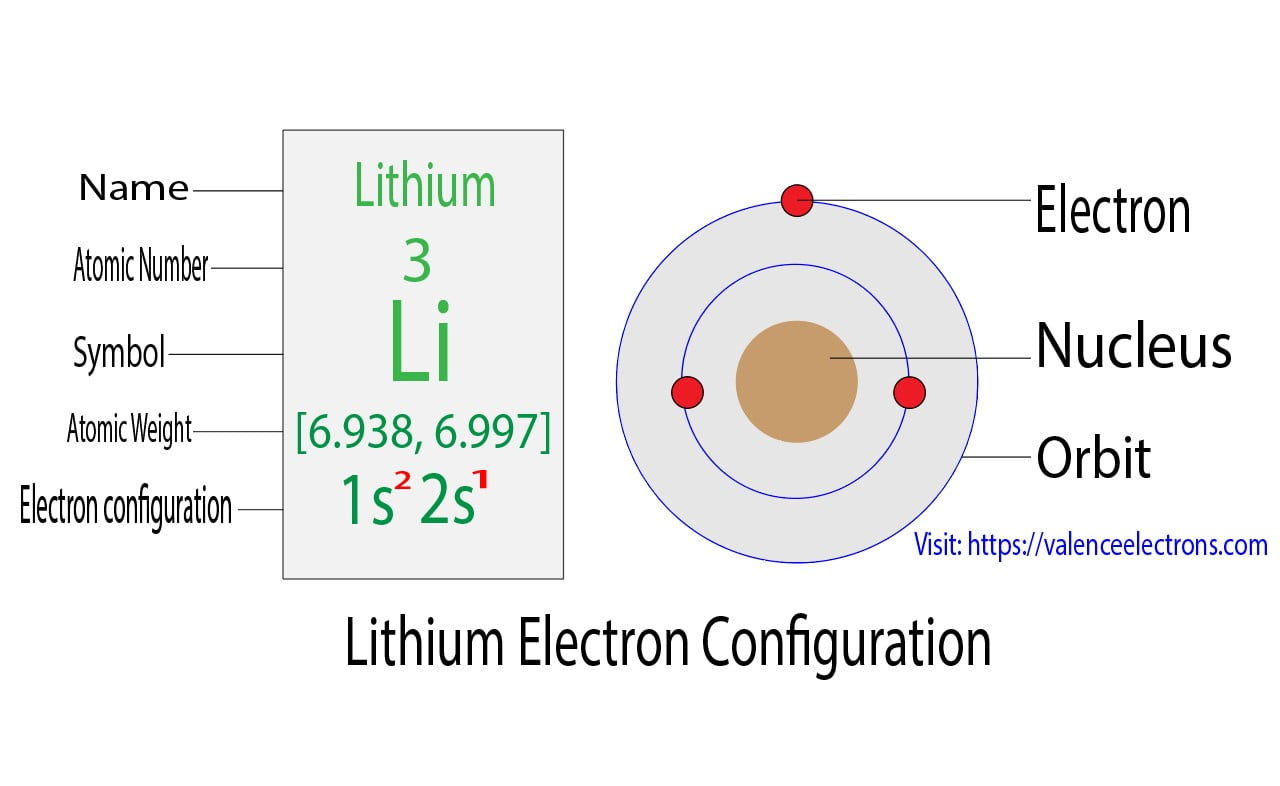
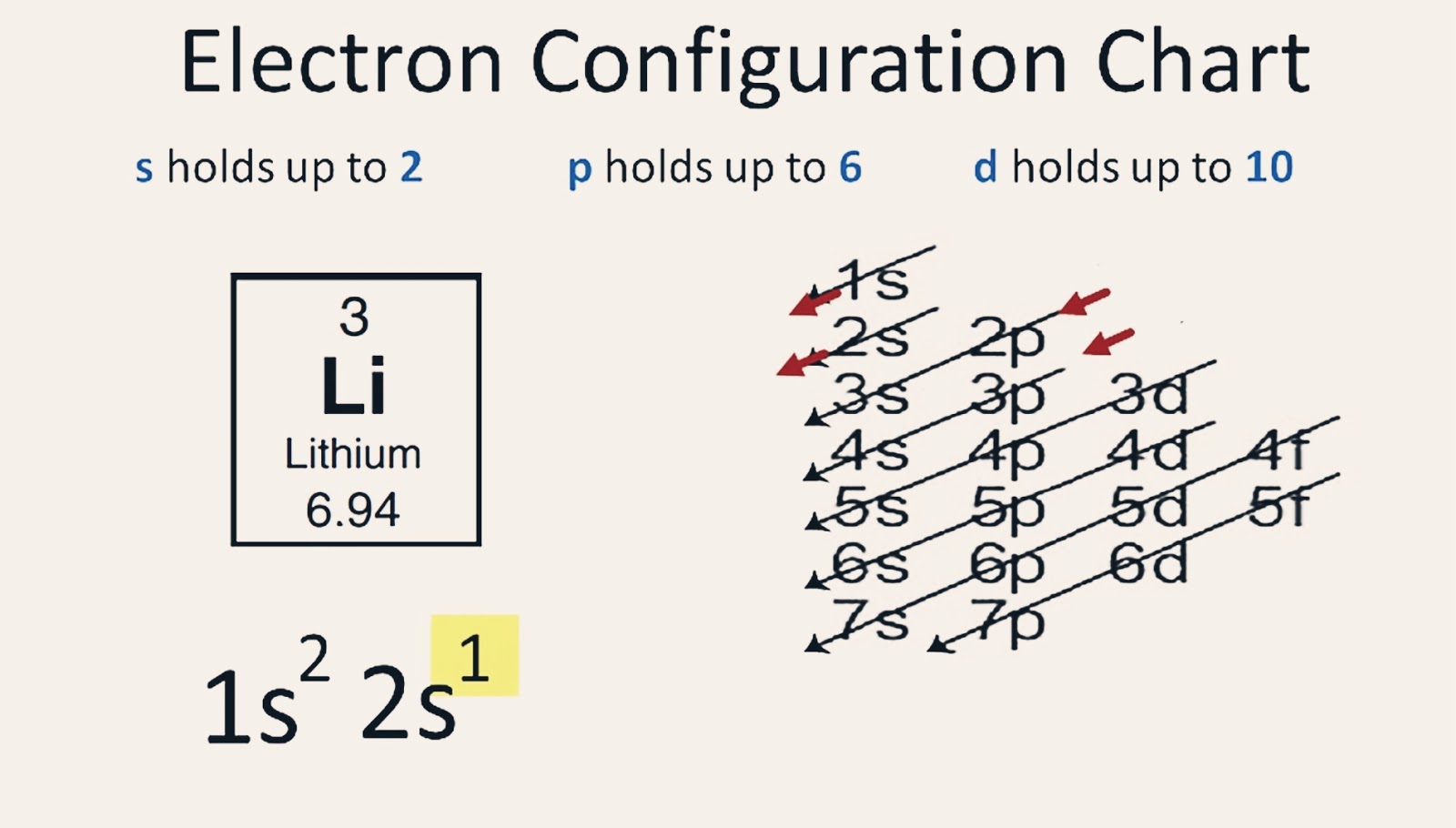
Lithium Electron Configuration Long Form
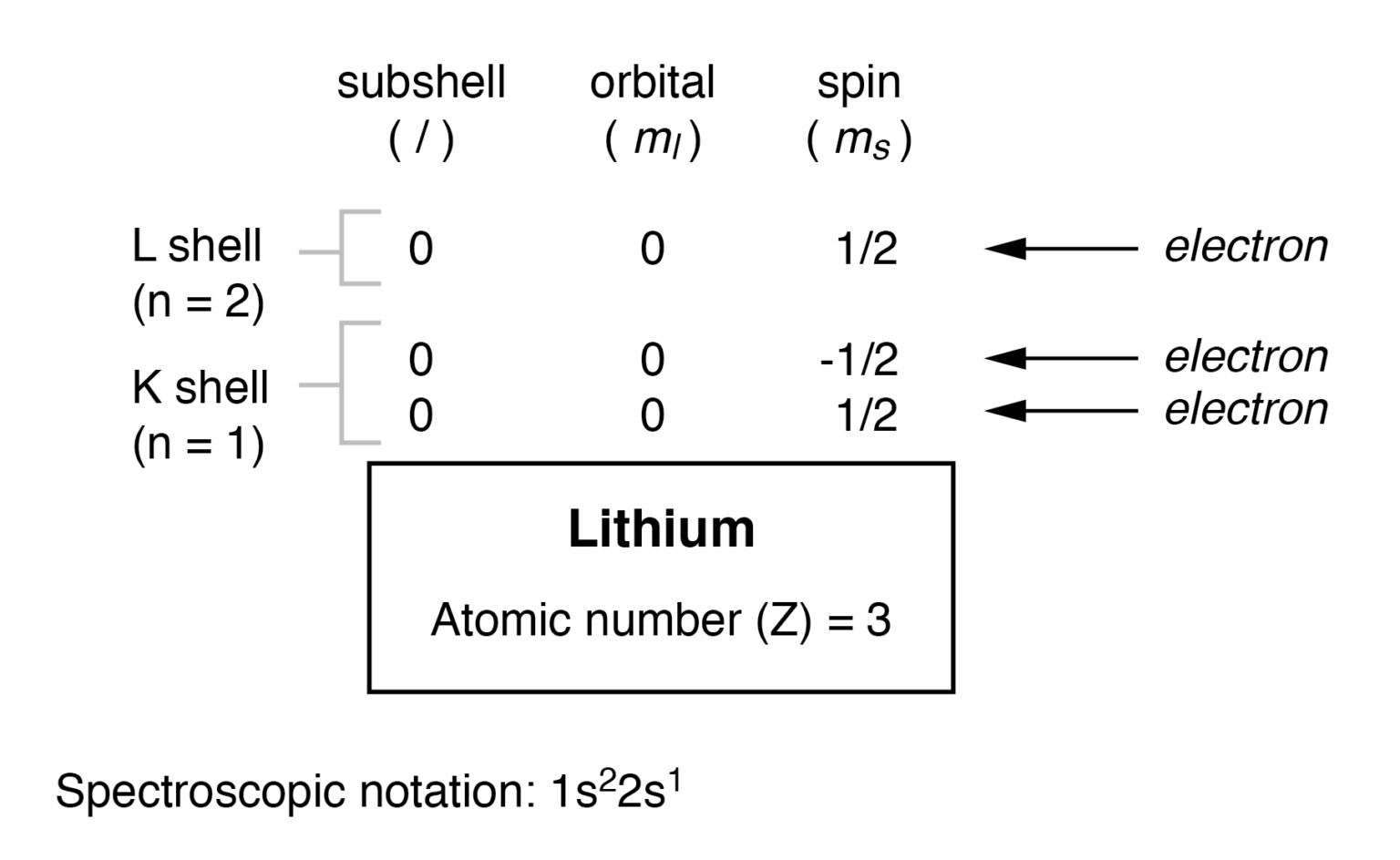
Lithium Electron Configuration Long Form - The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. To write the electron configuration for lithium, the first two electrons enter the 1s orbital. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. Since 1s can hold only two electrons, the rest of. View rotating bohr models for all 118.
Access detailed info on all elements: Atomic mass, electron configurations, charges, and more. Since 1s can hold only two electrons, the rest of. View rotating bohr models for all 118. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. To write the electron configuration for lithium, the first two electrons enter the 1s orbital.
Since 1s can hold only two electrons, the rest of. To write the electron configuration for lithium, the first two electrons enter the 1s orbital. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. View rotating bohr models for all 118.
Electron Configuration Of Lithium
To write the electron configuration for lithium, the first two electrons enter the 1s orbital. Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. View rotating bohr models for all 118. Atomic mass, electron configurations, charges, and more. Since 1s can hold only two electrons, the rest of.
Lithium Element With Reactions, Properties, Uses, & Price Periodic Table
Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. Access detailed info on all elements: Since 1s can hold only two electrons, the rest of. To write the electron configuration for lithium, the first two electrons enter the 1s orbital. View rotating bohr models for all 118.
How Do We Can Find A Electron Configuration For Lithium
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Since 1s can hold only two electrons, the rest of. Access detailed info on all elements: Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron..
electronarrangementforlithiumatom TechnoCrazed
View rotating bohr models for all 118. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. To write the electron configuration for lithium, the first.
14+ Lithium Lewis Dot Structure Robhosking Diagram
Since 1s can hold only two electrons, the rest of. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Access detailed info on all elements: To write the electron configuration for lithium, the first two electrons enter the 1s orbital. View rotating bohr models for all.
Electron configurations
Since 1s can hold only two electrons, the rest of. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Access detailed info on all elements: To write the electron configuration for lithium, the first two electrons enter the 1s orbital. Now, the lithium cation, li+, is.
Lithium electron configuration Stock Image C029/5021 Science
Access detailed info on all elements: Since 1s can hold only two electrons, the rest of. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. View rotating bohr models for all 118. Now, the lithium cation, li+, is formed when lithium loses the electron located on.
Lithium Atom Diagram / Atomic Structure Chemistry 10 A lithium atom
To write the electron configuration for lithium, the first two electrons enter the 1s orbital. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. View rotating bohr models for all 118. Since 1s can hold only two electrons, the rest of. Now, the lithium cation, li+,.
How to Write the Electron Configuration for Lithium?
Now, the lithium cation, li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. Since 1s can hold only two electrons, the rest of. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. To write the electron configuration for.
【5 Steps】Electron Configuration of Lithium(Li) in Just 5 Steps
Atomic mass, electron configurations, charges, and more. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Access detailed info on all elements: View rotating bohr models for all 118. Since 1s can hold only two electrons, the rest of.
Access Detailed Info On All Elements:
Since 1s can hold only two electrons, the rest of. To write the electron configuration for lithium, the first two electrons enter the 1s orbital. View rotating bohr models for all 118. Atomic mass, electron configurations, charges, and more.
Now, The Lithium Cation, Li+, Is Formed When Lithium Loses The Electron Located On Its Outermost Shell → Its Valence Electron.
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.

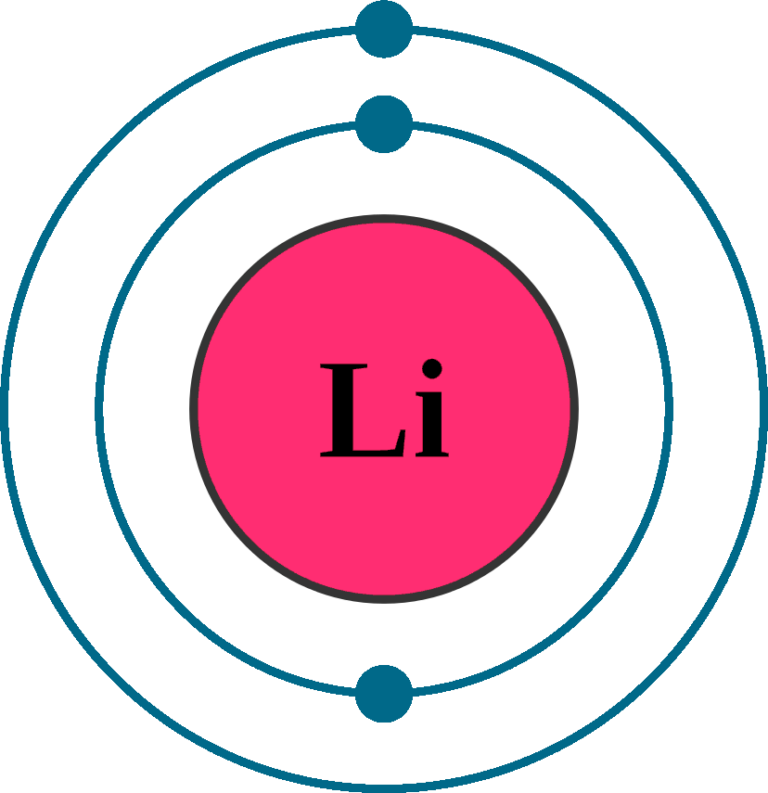

/lithiumatom-56a12c335f9b58b7d0bcc103.jpg)