In What Form Can An Ionic Compound Conduct Electricity
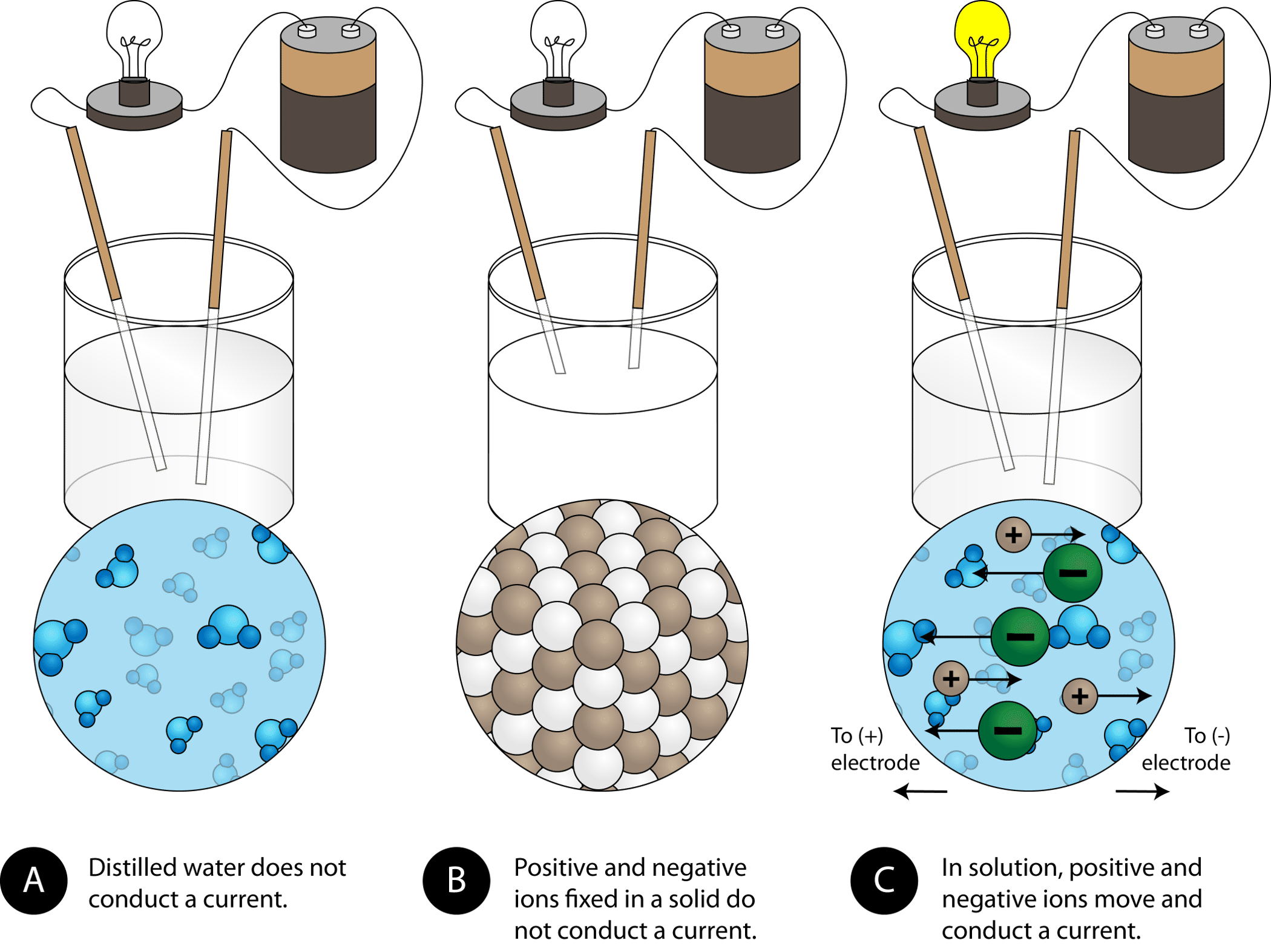
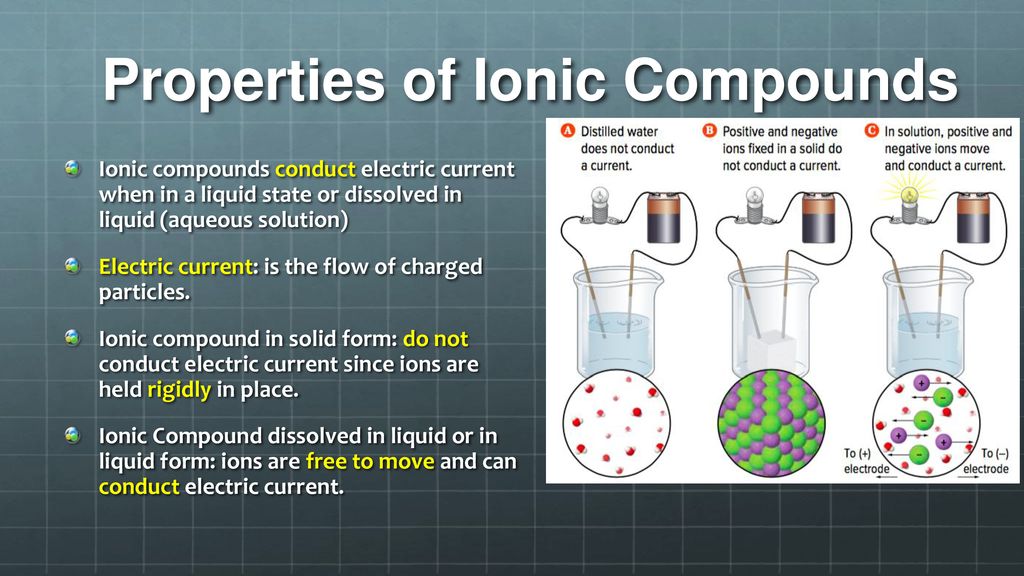
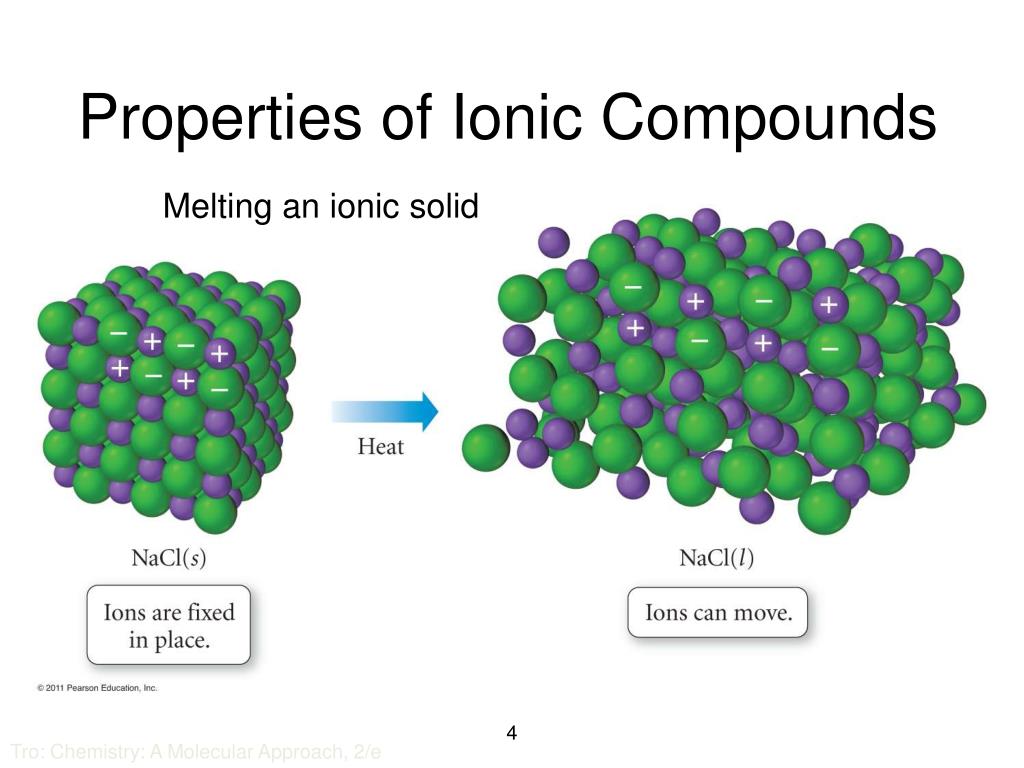
In What Form Can An Ionic Compound Conduct Electricity - Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ionic compounds conduct electricity when melted or in solution. Learn about and revise ionic compounds. They are insulators when solid. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of.
Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds conduct electricity when melted or in solution. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. They are insulators when solid. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Learn about and revise ionic compounds.
Ionic compounds conduct electricity when melted or in solution. They are insulators when solid. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Learn about and revise ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the.
Ionic Properties
Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Learn about and revise ionic compounds. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid..
Would The Resulting Solution Conduct Electricity SAEQVU
Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Learn about and revise ionic compounds. Ionic compounds conduct electricity when melted or in solution. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ions of opposite charges are tightly packed together to create.
Ionic Bonding. ppt download
In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ionic compounds conduct electricity when melted or in solution. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. They are insulators when solid. Ionic compounds conducts electricity only in molten state as the.
Properties of Ionic Compounds GCSE Chemistry Revision
Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Learn about and revise ionic compounds. In short, ionic compounds.
How Ionic Compounds Conduct Electricity GCSE Chemistry kayscience
Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. Learn about and revise ionic compounds. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ionic compounds conduct electricity when melted or in solution. Ions of opposite charges are tightly packed together to create crystalline.
What Is An Ionic Compound? Formula and Defination
Ionic compounds conduct electricity when melted or in solution. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ions of opposite charges are tightly packed together to create crystalline solids.
Properties of ionic compounds Chemistry Quizizz
Learn about and revise ionic compounds. Ionic compounds conduct electricity when melted or in solution. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. They are insulators when solid. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the.
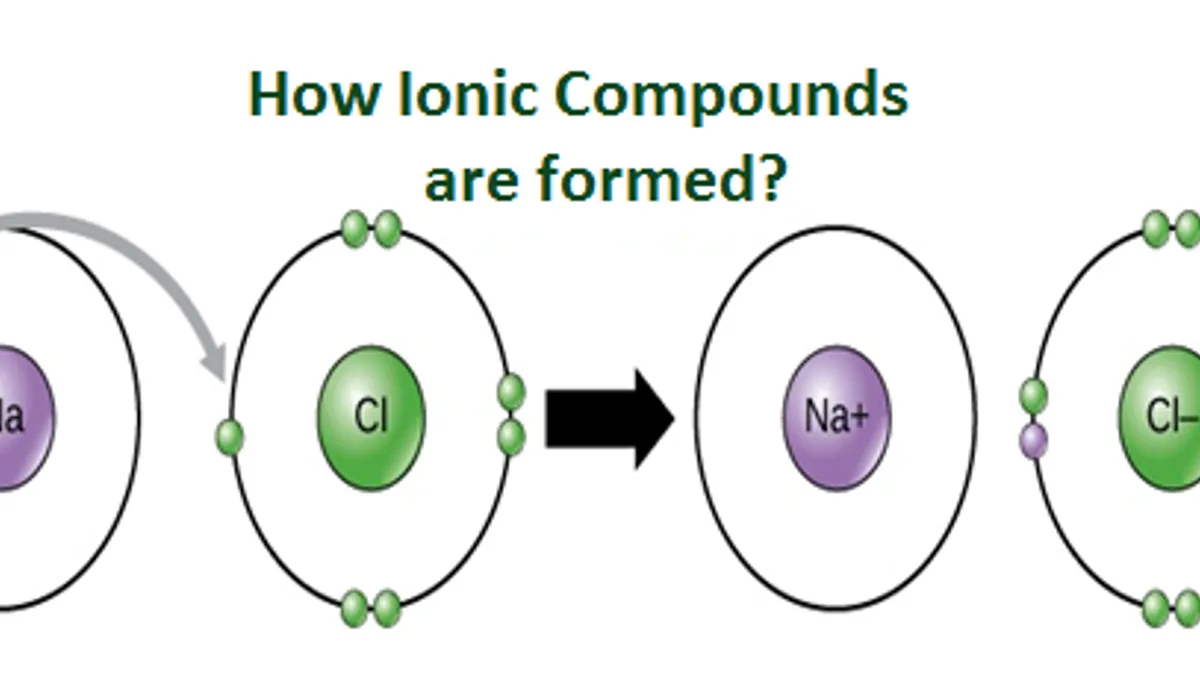
What are Ionic Compounds and how they are formed?
Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds conduct electricity when melted or in solution. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. They are insulators when solid. Learn about and revise ionic compounds.
Ionic Bond — Formation & Compounds Expii
Ionic compounds conduct electricity when melted or in solution. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. In.
How Do Ions Conduct Electricity
Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. Ionic compounds conducts electricity only in molten state as the ions are held together by strong electrostatic forces of. They are insulators when solid. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds. Learn about and revise ionic.
Ionic Compounds Conducts Electricity Only In Molten State As The Ions Are Held Together By Strong Electrostatic Forces Of.
Ionic compounds conduct electricity when melted or in solution. Ionic compounds are conductors of electricity when molten or in solution and insulators when solid. In short, ionic compounds conduct electricity in water because they separate into charged ions, which are then attracted to the. Ions of opposite charges are tightly packed together to create crystalline solids known as ionic compounds.
Learn About And Revise Ionic Compounds.
They are insulators when solid.