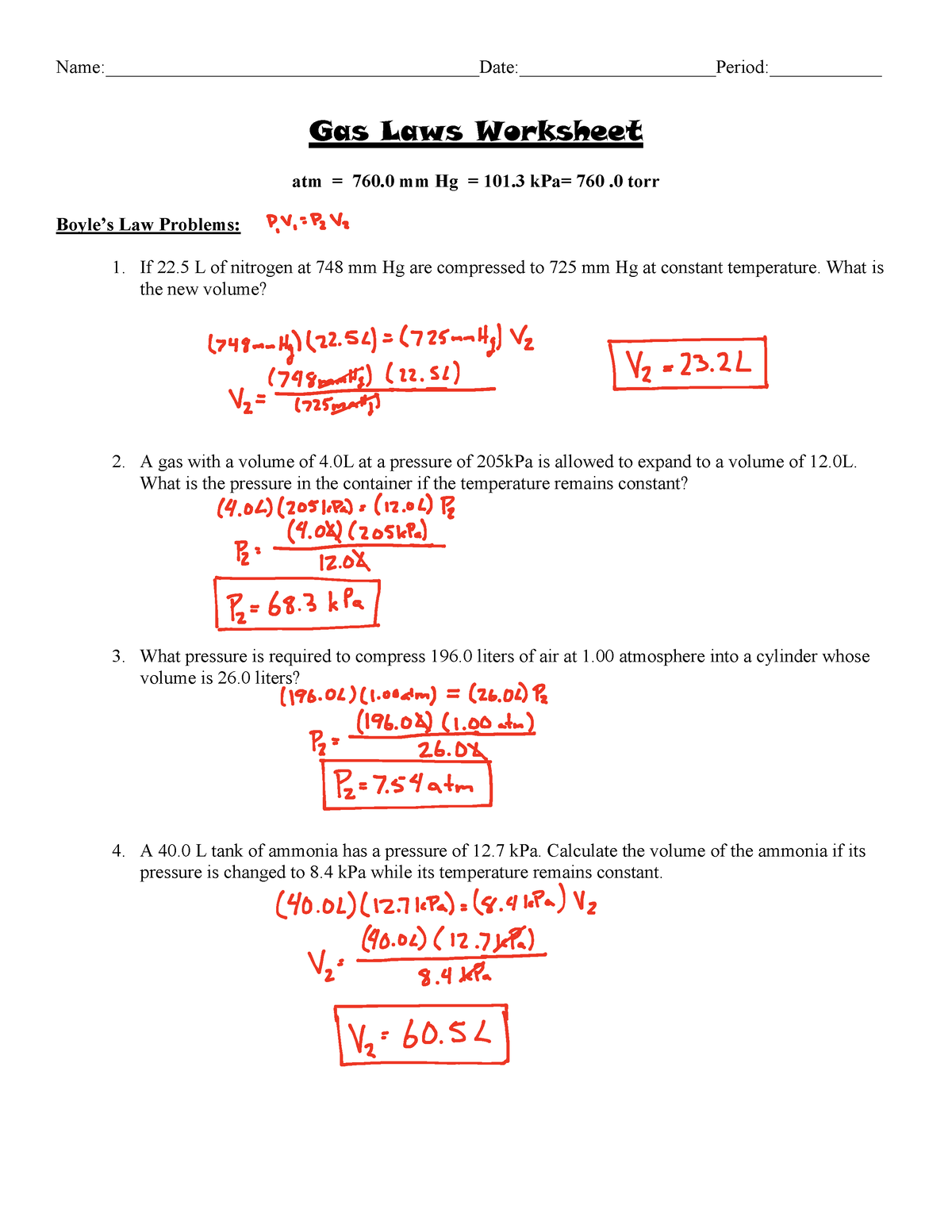
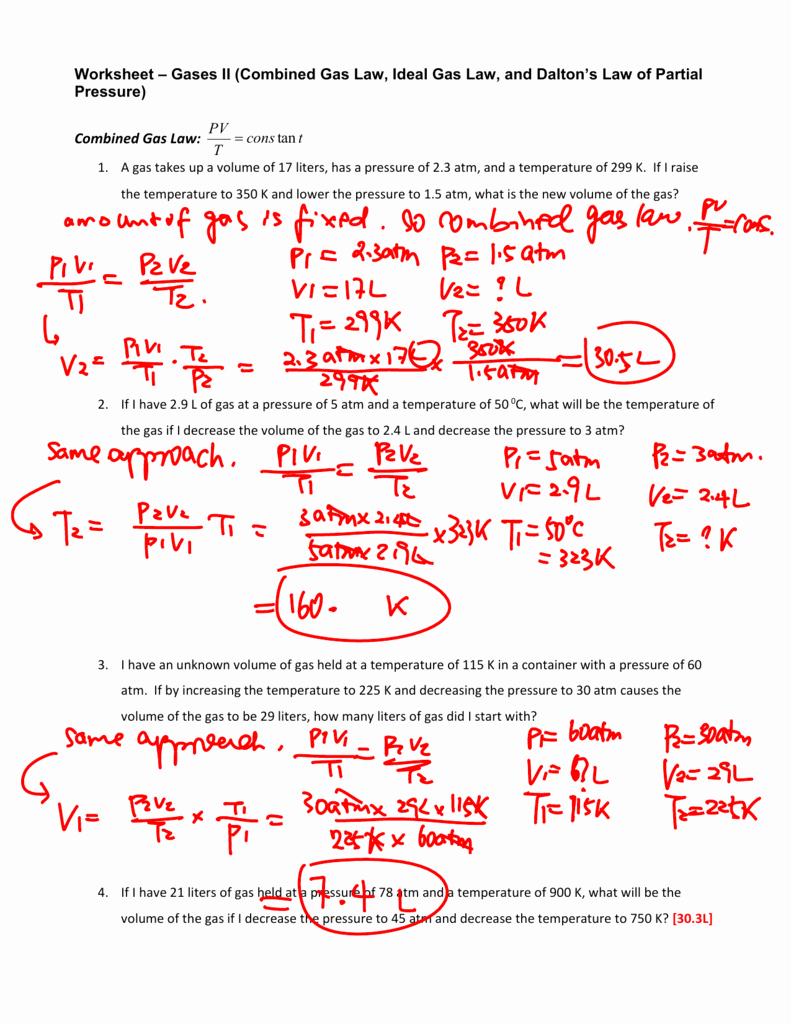
Ideal Gas Law Worksheet Answers
Ideal Gas Law Worksheet Answers - The ideal gas law directions: 2) at what temperature would 2.10 moles of n2 gas have a. Assume that the lungs are at. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 1) given the following sets of values, calculate the unknown quantity. Show your work, including proper units, to earn full credit. Solve each of the following problems. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l?
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Solve each of the following problems. The ideal gas law directions: 2) at what temperature would 2.10 moles of n2 gas have a. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. Show your work, including proper units, to earn full credit. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 1) given the following sets of values, calculate the unknown quantity. Assume that the lungs are at.
The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 2) at what temperature would 2.10 moles of n2 gas have a. The ideal gas law directions: Show your work, including proper units, to earn full credit. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. Assume that the lungs are at. 1) given the following sets of values, calculate the unknown quantity. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Solve each of the following problems.
Ideal Gas Law Worksheet 144 Answer Key Greenus
The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The ideal gas law directions:.
Gas Laws Worksheet answer key Gas Laws Worksheet atm = 760 mm Hg
Assume that the lungs are at. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 1) given the following sets of values, calculate the unknown quantity. Solve each of the following problems. Show your work, including proper units, to earn full credit.
Ideal Gas Law Worksheet Answers
1) given the following sets of values, calculate the unknown quantity. Solve each of the following problems. The ideal gas law directions: Show your work, including proper units, to earn full credit. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal.
Ideal Gas Law Problems Worksheet Worksheets For Kindergarten
Solve each of the following problems. Assume that the lungs are at. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles.
Ideal Gas Law Worksheet PV = nRT
Assume that the lungs are at. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The ideal gas law directions: Show your work, including proper units, to earn full credit. 2) at what temperature would.
Ideal Gas Law Questions And Answers
Show your work, including proper units, to earn full credit. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 2) at what temperature would 2.10 moles of n2 gas have a. Solve each of the following problems. 1) given the following sets of values, calculate the unknown quantity.
SOLUTION Ideal gass law worksheet Studypool
The ideal gas law directions: Assume that the lungs are at. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Solve each of the following problems. Show your work, including proper units, to earn full credit.
Ideal Gas Law Worksheet Science ShowMe
The ideal gas law directions: 1) given the following sets of values, calculate the unknown quantity. 2) at what temperature would 2.10 moles of n2 gas have a. Show your work, including proper units, to earn full credit. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal.
Ideal Gas Law Worksheet 2 Answer Ideal Gas Law Worksheet PV = nRT Use
The ideal gas law directions: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 2) at what temperature would 2.10 moles of n2 gas have a. Solve each of the following problems. How many moles.
Ideal Gas Law Practice With Answers
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 1) given the following sets of values, calculate the unknown quantity. Show your work, including proper units, to earn full credit. Assume that the lungs are at. Solve each of the following problems.
Solve Each Of The Following Problems.
1) given the following sets of values, calculate the unknown quantity. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 2) at what temperature would 2.10 moles of n2 gas have a. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l?
Show Your Work, Including Proper Units, To Earn Full Credit.
The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. Assume that the lungs are at. The ideal gas law directions: