Homogeneous Vs Heterogeneous Mixture
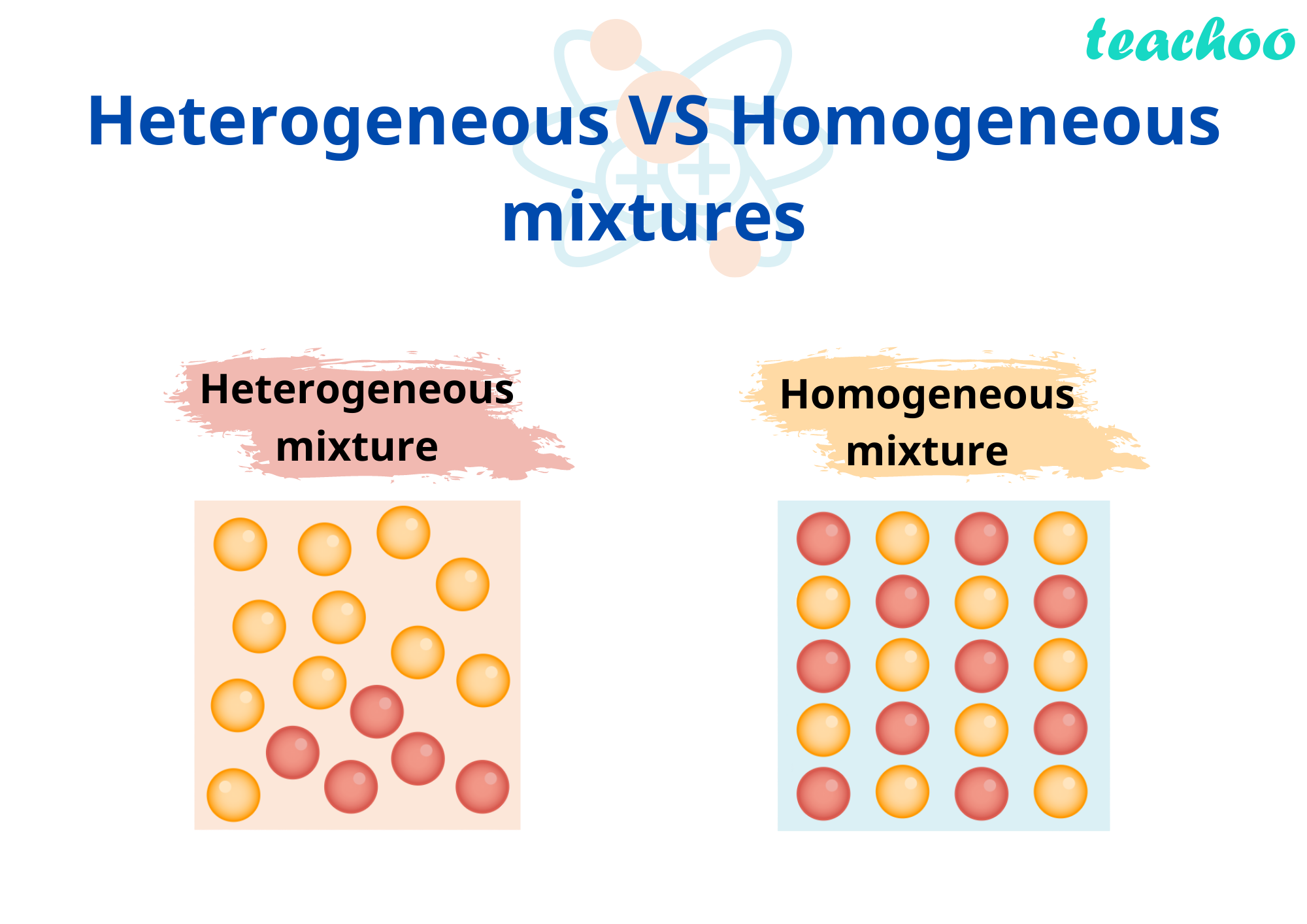
Homogeneous Vs Heterogeneous Mixture - Homogeneous mixtures are sources of water,. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new substance (salt. A homogeneous solution tends to be identical, no matter how you sample it. By combining two or more substances, a mixture is produced. Mixtures are of two types: Individual substances that constitute a homogeneous. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. A homogeneous mixture has a uniform composition and appearance. There are two types of mixtures: In homogeneous mixture, components are uniformly distributed and not easily distinguishable.
The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. Individual substances that constitute a homogeneous. A homogeneous solution tends to be identical, no matter how you sample it. A homogeneous mixture has a uniform composition and appearance. Homogeneous mixtures are sources of water,. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new substance (salt. There are two types of mixtures: In homogeneous mixture, components are uniformly distributed and not easily distinguishable. Mixtures are of two types: By combining two or more substances, a mixture is produced.
A homogeneous mixture has a uniform composition and appearance. By combining two or more substances, a mixture is produced. There are two types of mixtures: A homogeneous solution tends to be identical, no matter how you sample it. Individual substances that constitute a homogeneous. Mixtures are of two types: Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new substance (salt. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. Homogeneous mixtures are sources of water,. In homogeneous mixture, components are uniformly distributed and not easily distinguishable.
Homogeneous vs heterogeneous mixture physical properties outline
Individual substances that constitute a homogeneous. There are two types of mixtures: A homogeneous mixture has a uniform composition and appearance. A homogeneous solution tends to be identical, no matter how you sample it. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition.
Heterogeneous vs. Homogeneous Mixtures
A homogeneous mixture has a uniform composition and appearance. Individual substances that constitute a homogeneous. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new.
What is a Mixture in Chemistry? The Chemistry Blog
Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new substance (salt. By combining two or more substances, a mixture is produced. A homogeneous solution tends to be identical, no matter how you sample it. Individual substances that constitute a homogeneous. Homogeneous mixtures are sources of water,.
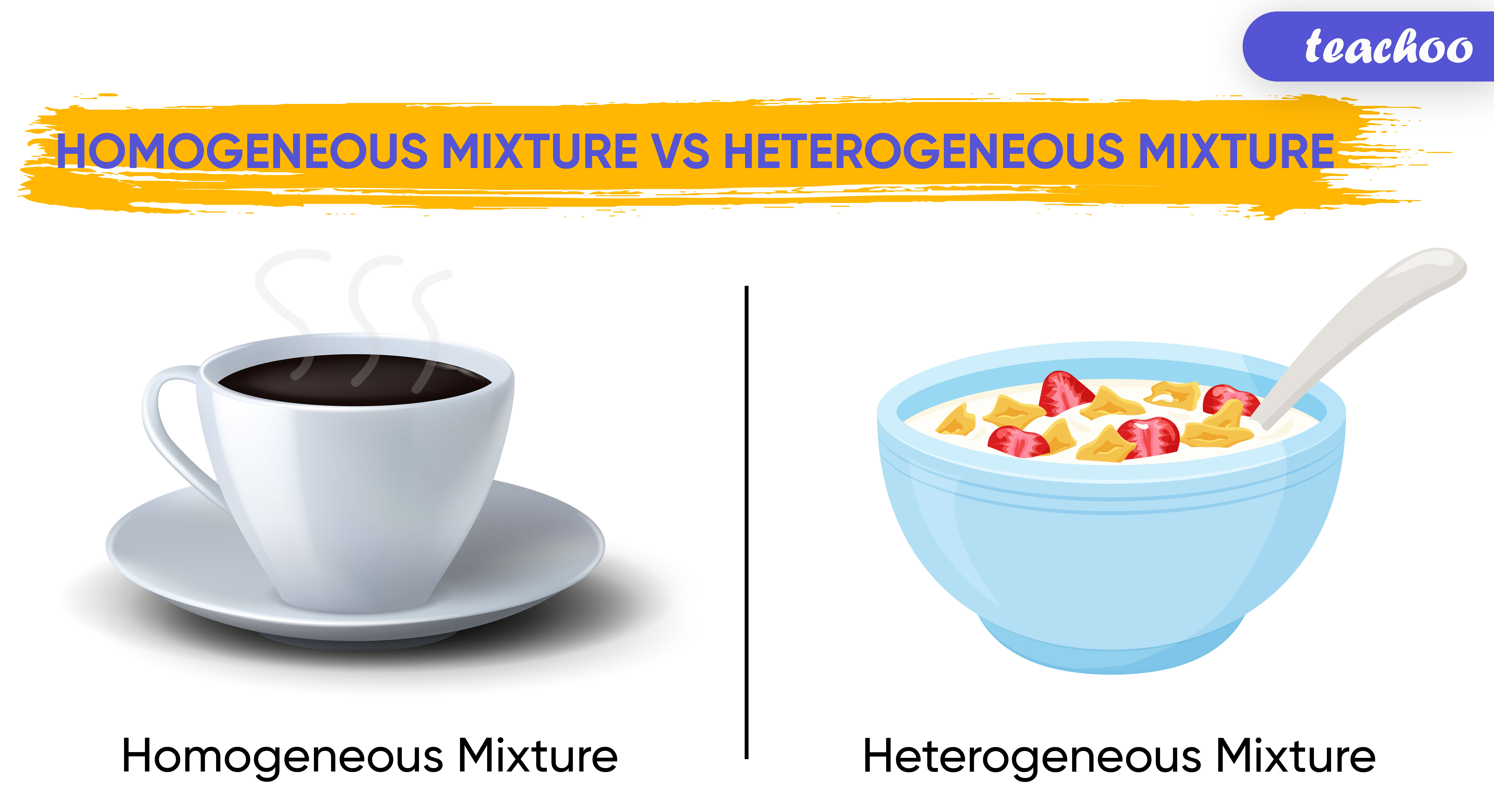
Differentiate b/w Homogeneous and Heterogeneous mixtures Teachoo
There are two types of mixtures: Mixtures are of two types: Individual substances that constitute a homogeneous. Homogeneous mixtures are sources of water,. A homogeneous mixture has a uniform composition and appearance.
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo
By combining two or more substances, a mixture is produced. Homogeneous mixtures are sources of water,. Individual substances that constitute a homogeneous. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. A homogeneous mixture has a uniform composition and appearance.
Types of mixture Homogeneous and Heterogeneous — lesson. Science State
In homogeneous mixture, components are uniformly distributed and not easily distinguishable. Individual substances that constitute a homogeneous. By combining two or more substances, a mixture is produced. A homogeneous solution tends to be identical, no matter how you sample it. Mixtures are of two types:
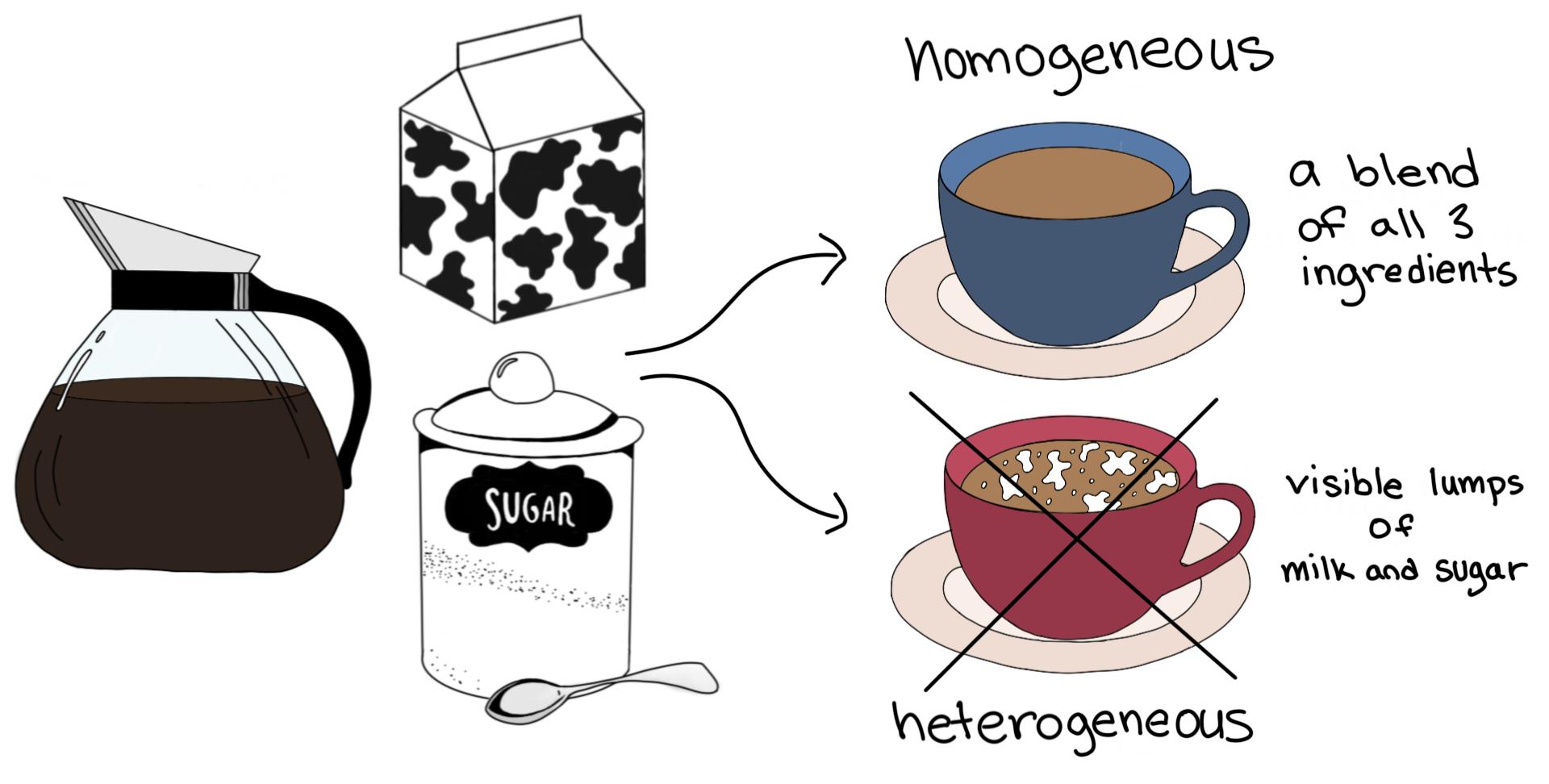
Homogeneous vs. Heterogeneous Mixtures — A Comparison Expii
The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. In homogeneous mixture, components are uniformly distributed and not easily distinguishable. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new substance (salt. There are.
Difference Between Homogeneous and Heterogeneous Mixtures
By combining two or more substances, a mixture is produced. Individual substances that constitute a homogeneous. A homogeneous solution tends to be identical, no matter how you sample it. Mixtures are of two types: In homogeneous mixture, components are uniformly distributed and not easily distinguishable.
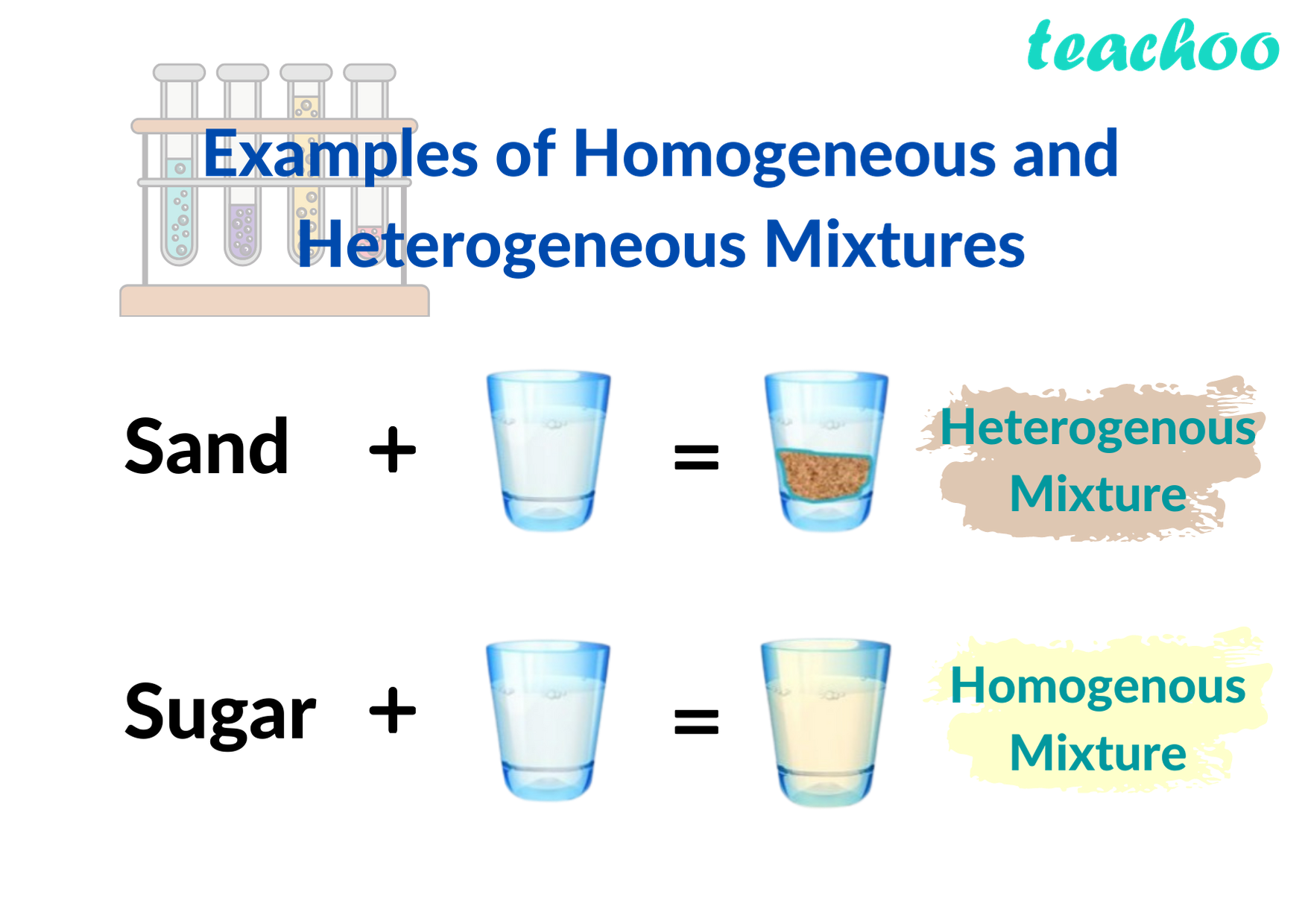
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo
In homogeneous mixture, components are uniformly distributed and not easily distinguishable. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition. Mixtures are of two types: A homogeneous solution tends to be identical, no matter how you sample it. By combining two or more substances, a mixture is.
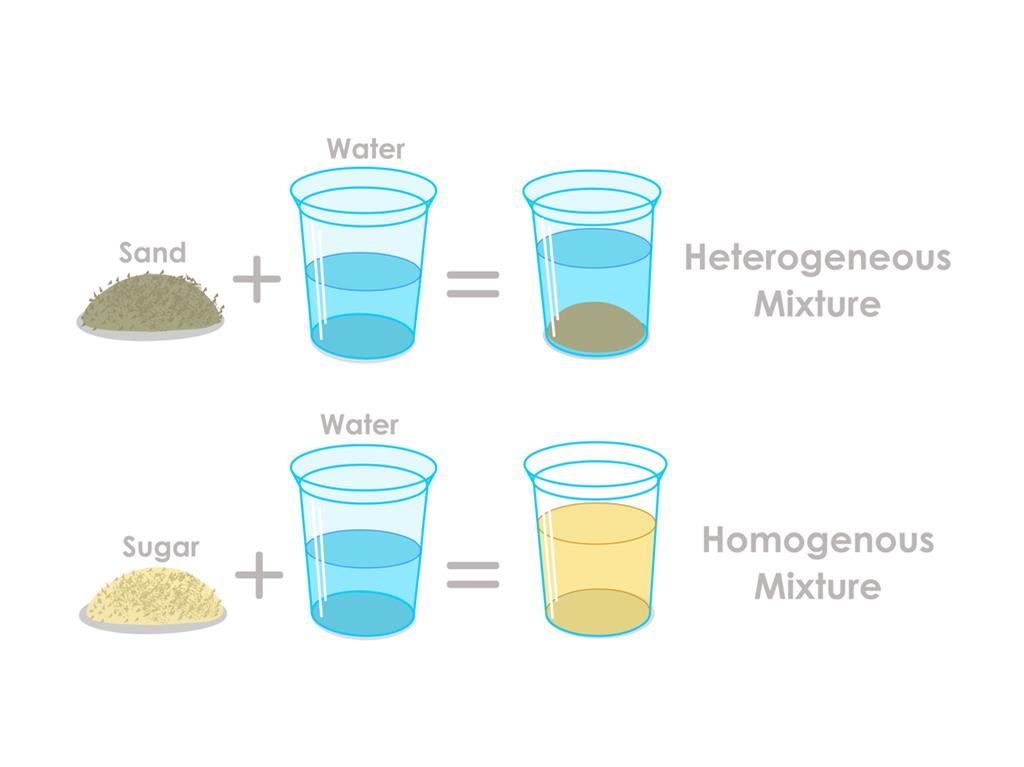
10 Examples of Mixtures
Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new substance (salt. By combining two or more substances, a mixture is produced. Mixtures are of two types: Individual substances that constitute a homogeneous. In homogeneous mixture, components are uniformly distributed and not easily distinguishable.
A Homogeneous Mixture Has A Uniform Composition And Appearance.
There are two types of mixtures: By combining two or more substances, a mixture is produced. Individual substances that constitute a homogeneous. Scientifically speaking, a homogeneous mixture is one in which different parts (such as salt and water) have been uniformly combined into a new substance (salt.
Homogeneous Mixtures Are Sources Of Water,.
In homogeneous mixture, components are uniformly distributed and not easily distinguishable. A homogeneous solution tends to be identical, no matter how you sample it. Mixtures are of two types: The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed and the uniformity of their composition.

/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)