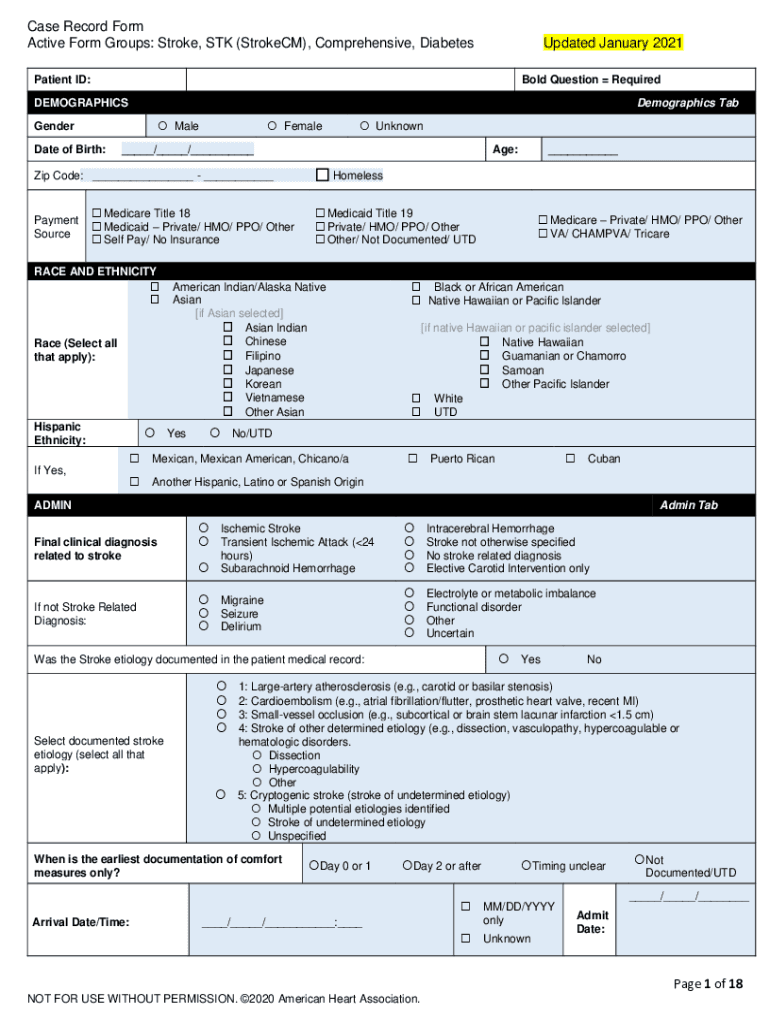
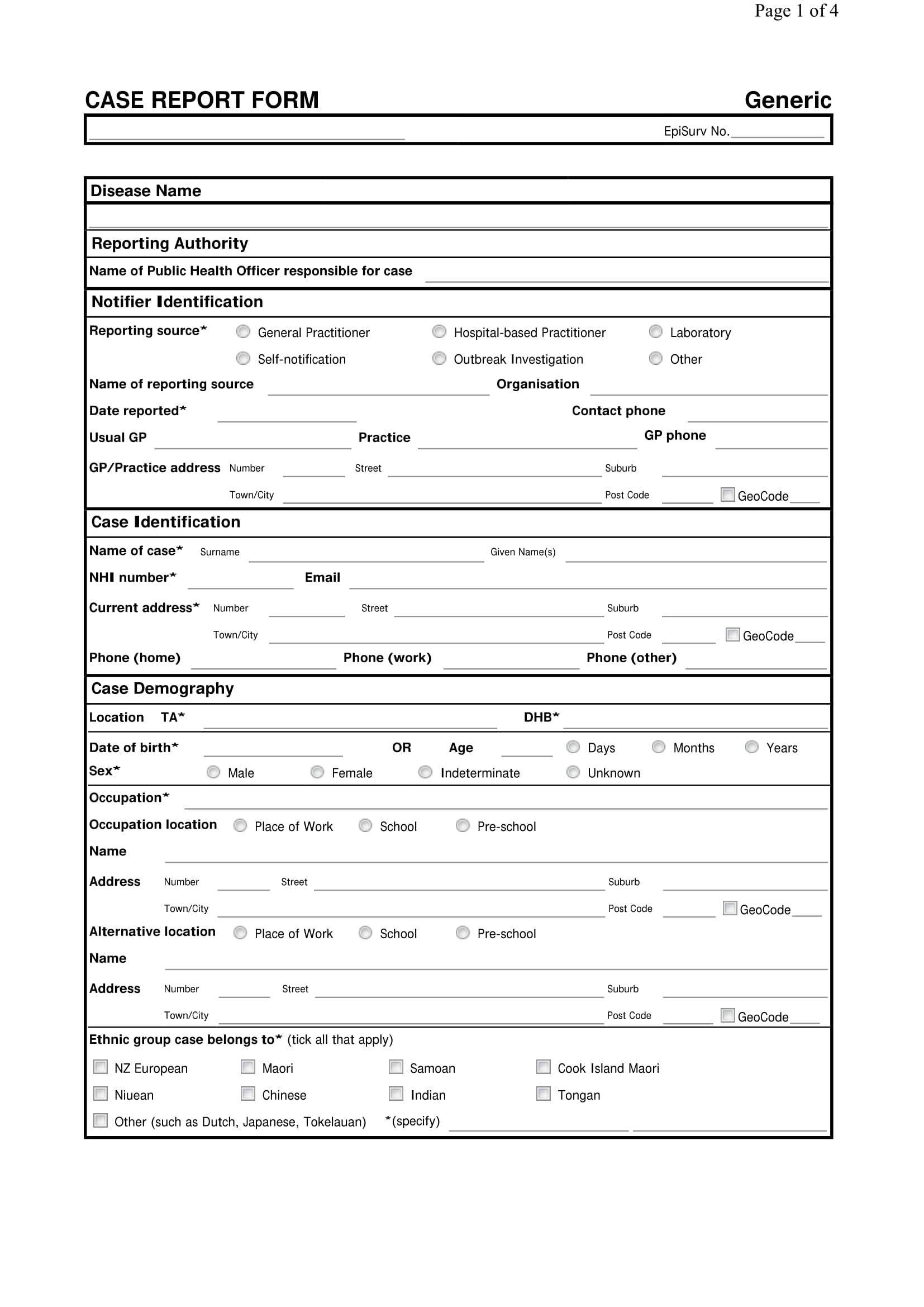
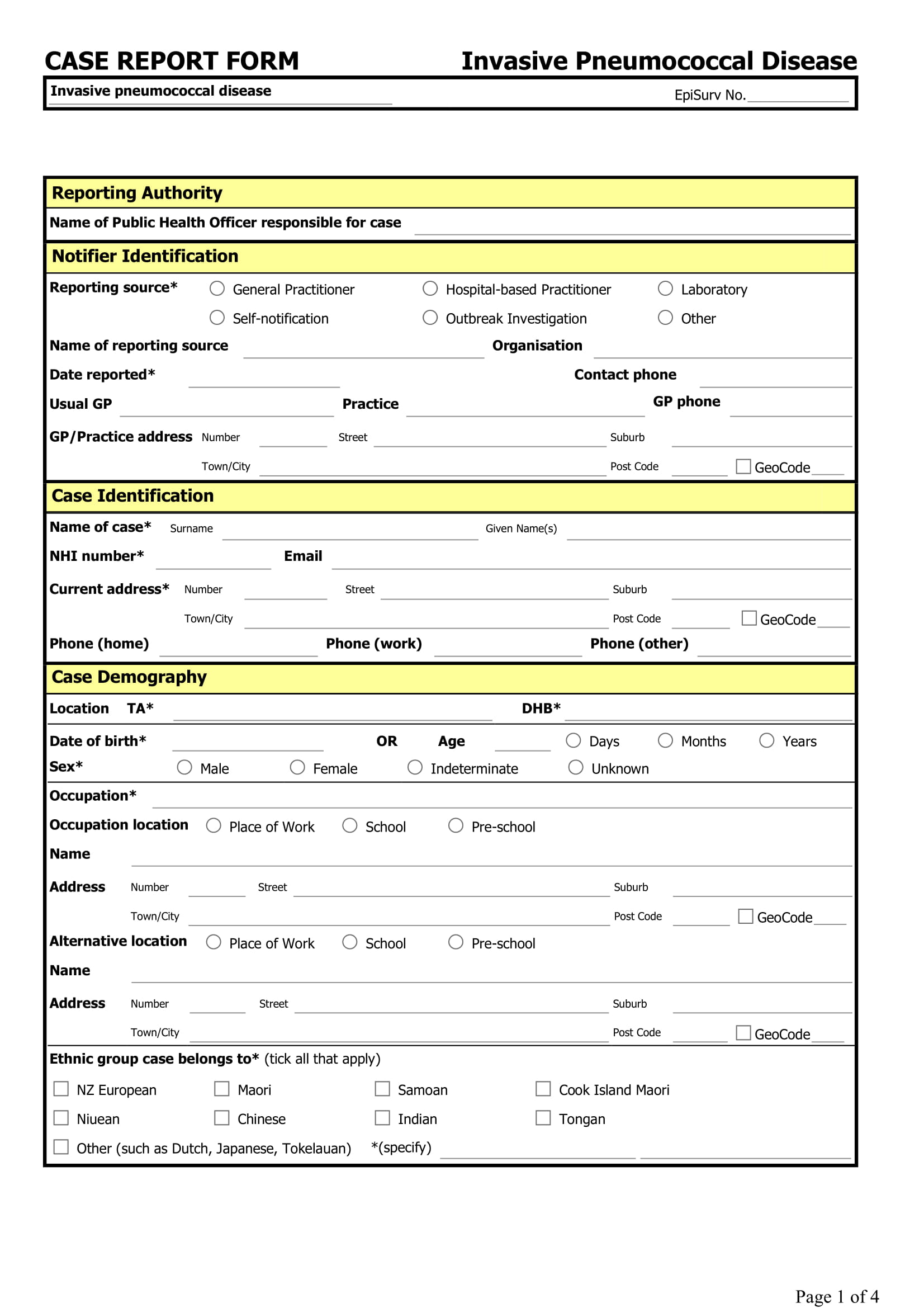
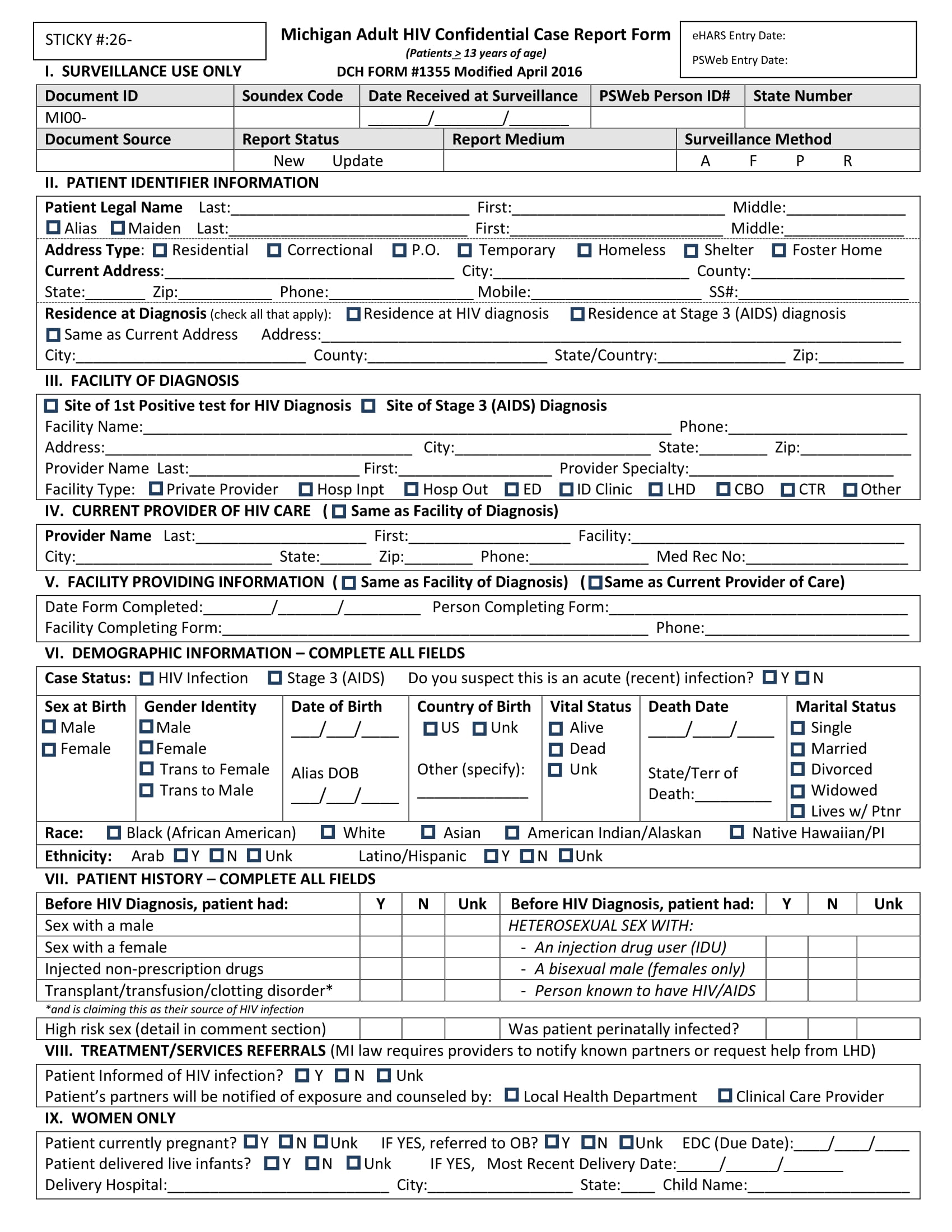
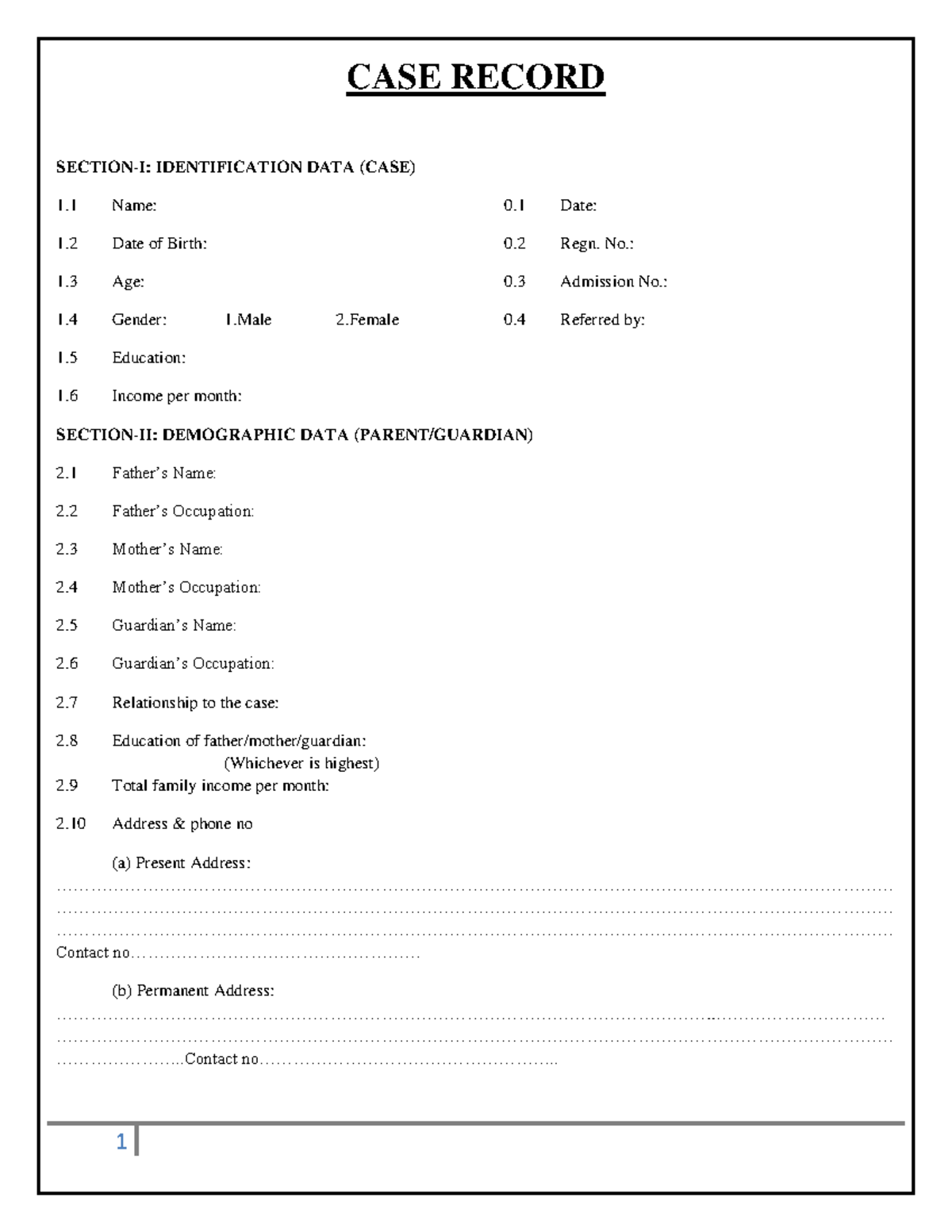
Case Record Form
Case Record Form - Case report forms (crfs) are critical documents in clinical research that record study data for each participant. This presentation covers the key elements,. Find out the advantages and disadvantages of paper. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Learn what a case record form (crf) is, how to design it, and what elements to include in it. Learn how to design and develop a reliable and valid case report form (crf) for clinical research. Careful design of crfs is crucial for. Find out the minimum information to.
Find out the minimum information to. Careful design of crfs is crucial for. Learn how to design and develop a reliable and valid case report form (crf) for clinical research. Find out the advantages and disadvantages of paper. This presentation covers the key elements,. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Learn what a case record form (crf) is, how to design it, and what elements to include in it. Case report forms (crfs) are critical documents in clinical research that record study data for each participant.
Find out the minimum information to. Case report forms (crfs) are critical documents in clinical research that record study data for each participant. Careful design of crfs is crucial for. This presentation covers the key elements,. Learn what a case record form (crf) is, how to design it, and what elements to include in it. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Find out the advantages and disadvantages of paper. Learn how to design and develop a reliable and valid case report form (crf) for clinical research.
Fillable Online Case Record Form Active Form Groups Stroke, Diabetes
This presentation covers the key elements,. Find out the advantages and disadvantages of paper. Find out the minimum information to. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Case report forms (crfs) are critical documents in clinical research that record study data for each participant.
Printable Botox Treatment Record Template Printable Word Searches
Find out the minimum information to. Learn what a case record form (crf) is, how to design it, and what elements to include in it. Case report forms (crfs) are critical documents in clinical research that record study data for each participant. Learn what a case report form (crf) is, how to complete it, and why it is important for.
FREE 15+ Case Report Forms in PDF MS Word
Find out the minimum information to. Find out the advantages and disadvantages of paper. This presentation covers the key elements,. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Learn how to design and develop a reliable and valid case report form (crf) for clinical research.
(PDF) CASE RECORD FORM INSTRUCTIONS SEVERE ACUTE … · This CRF is
Find out the advantages and disadvantages of paper. Careful design of crfs is crucial for. Learn how to design and develop a reliable and valid case report form (crf) for clinical research. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. This presentation covers the key elements,.
Case Record Form
Case report forms (crfs) are critical documents in clinical research that record study data for each participant. This presentation covers the key elements,. Careful design of crfs is crucial for. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Learn what a case record form (crf) is, how to.
Case Report Form ≡ Fill Out Printable PDF Forms Online
This presentation covers the key elements,. Find out the advantages and disadvantages of paper. Careful design of crfs is crucial for. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Find out the minimum information to.
FREE 15+ Case Report Forms in PDF MS Word
Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Find out the minimum information to. Case report forms (crfs) are critical documents in clinical research that record study data for each participant. Careful design of crfs is crucial for. Learn how to design and develop a reliable and valid.
Two pages of the case record form as an example. Download Scientific
This presentation covers the key elements,. Learn how to design and develop a reliable and valid case report form (crf) for clinical research. Find out the advantages and disadvantages of paper. Careful design of crfs is crucial for. Find out the minimum information to.
FREE 15+ Case Report Forms in PDF MS Word
Learn how to design and develop a reliable and valid case report form (crf) for clinical research. Find out the minimum information to. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. This presentation covers the key elements,. Careful design of crfs is crucial for.
CASE History b.ed CASE RECORD SECTIONI IDENTIFICATION DATA (CASE
Find out the advantages and disadvantages of paper. Find out the minimum information to. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Case report forms (crfs) are critical documents in clinical research that record study data for each participant. Learn how to design and develop a reliable and.
Careful Design Of Crfs Is Crucial For.
This presentation covers the key elements,. Learn what a case report form (crf) is, how to complete it, and why it is important for clinical trials. Learn how to design and develop a reliable and valid case report form (crf) for clinical research. Find out the advantages and disadvantages of paper.
Learn What A Case Record Form (Crf) Is, How To Design It, And What Elements To Include In It.
Find out the minimum information to. Case report forms (crfs) are critical documents in clinical research that record study data for each participant.