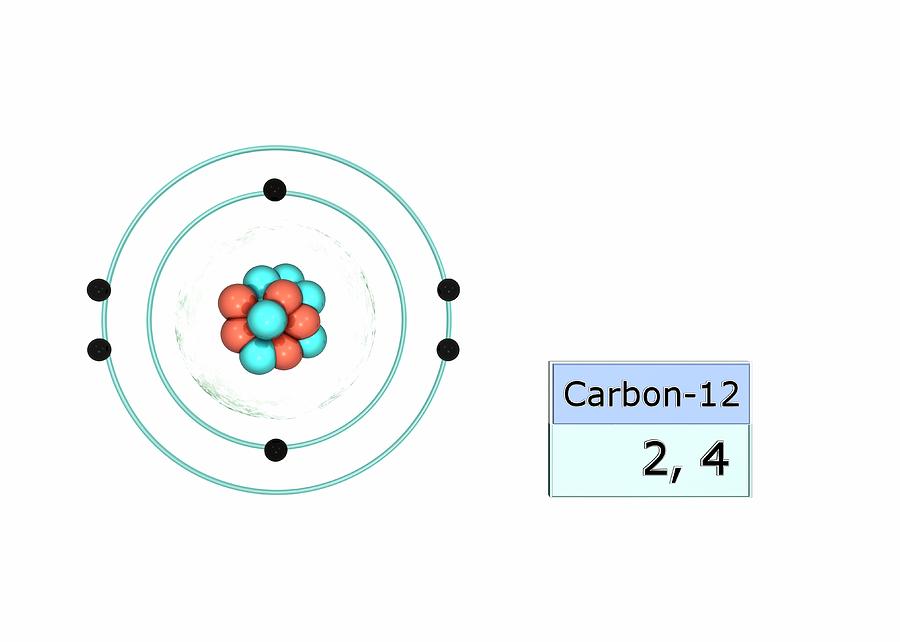
Carbon Electron Configuration Long Form
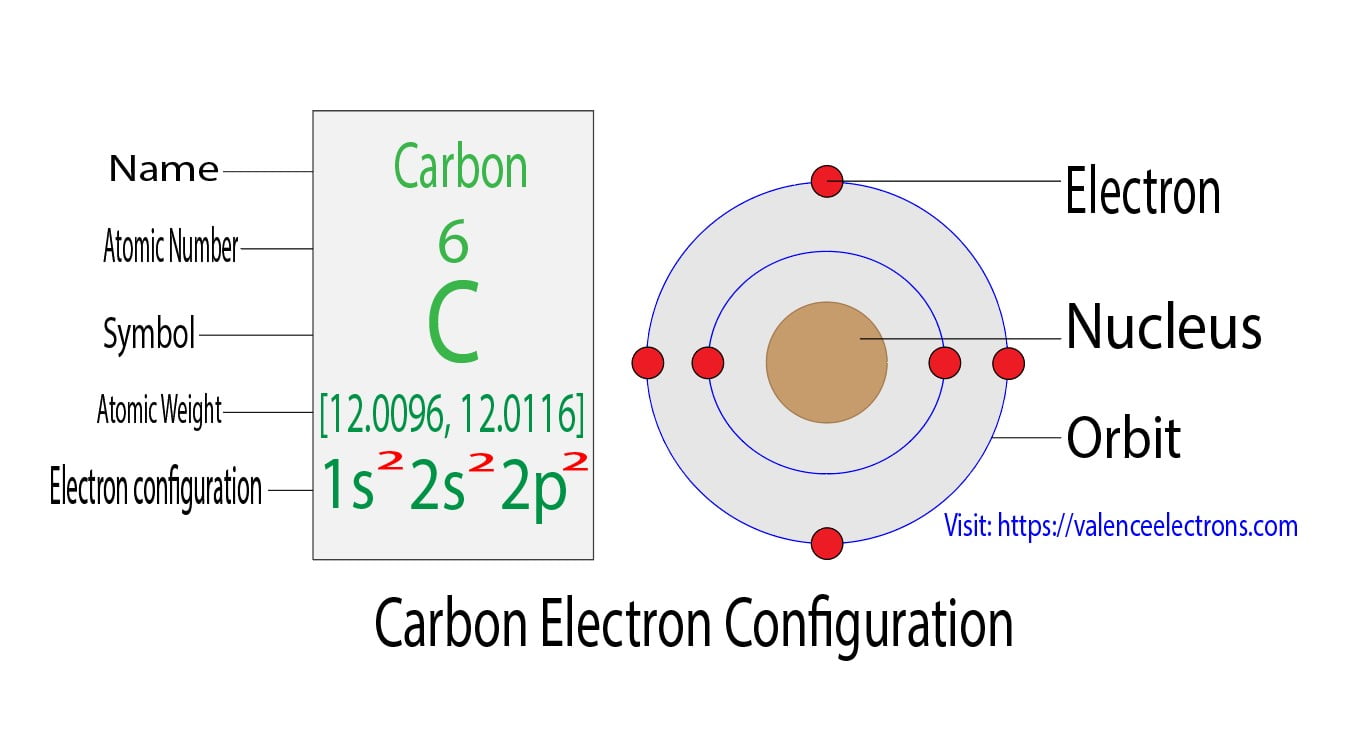
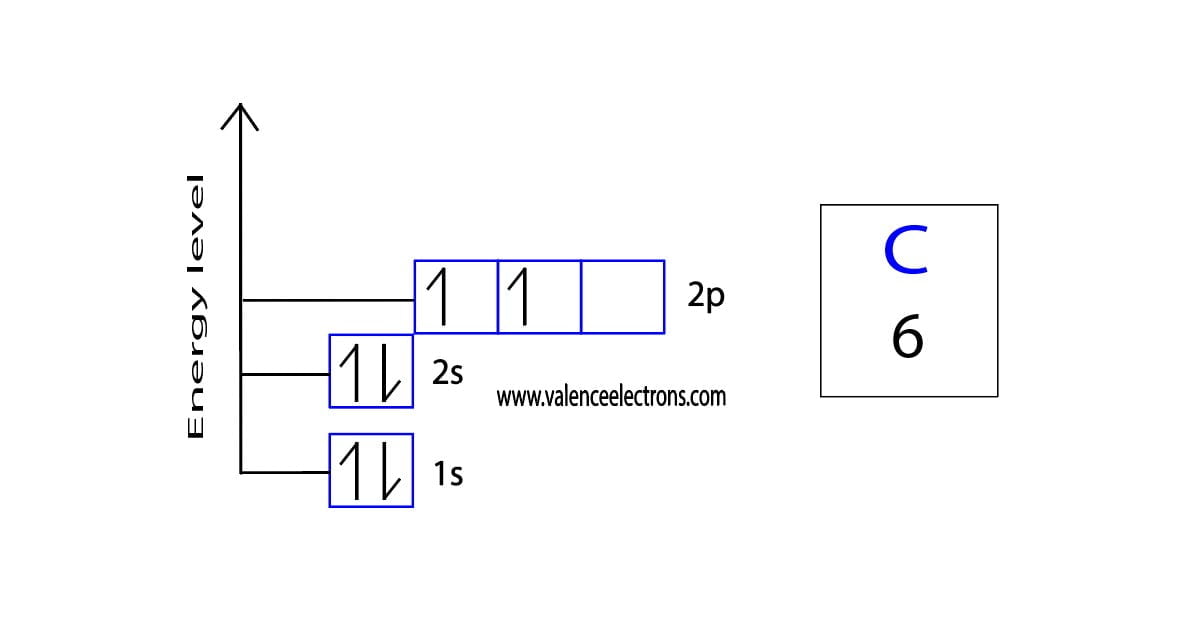
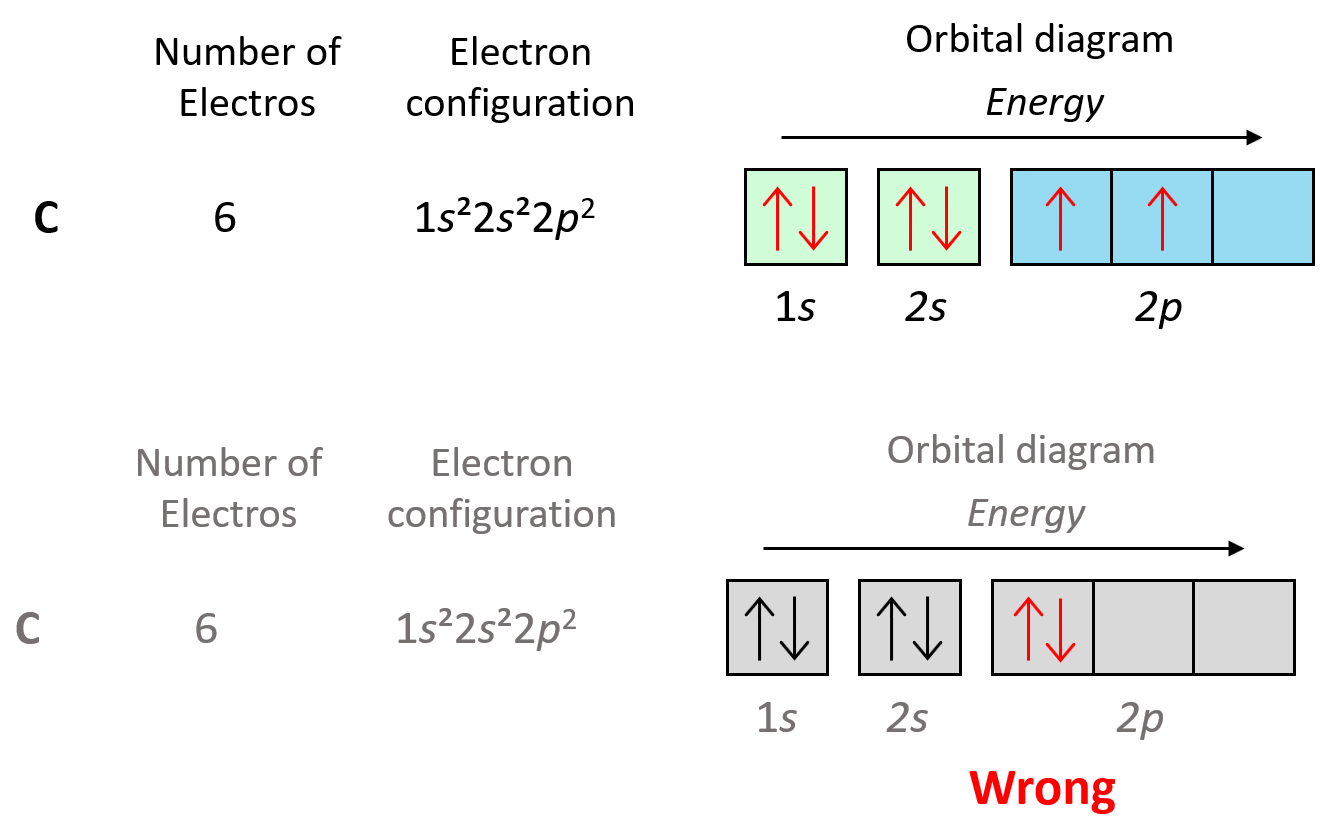
Carbon Electron Configuration Long Form - Access detailed info on all elements: Carbon’s electron configuration is written as 1s² 2s² 2p². It has six electrons in total. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Shell diagram of carbon (c). It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon, the sixth element in the periodic table, is. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118. The electronic configuration of a carbon atom is 1s² 2s² 2p².
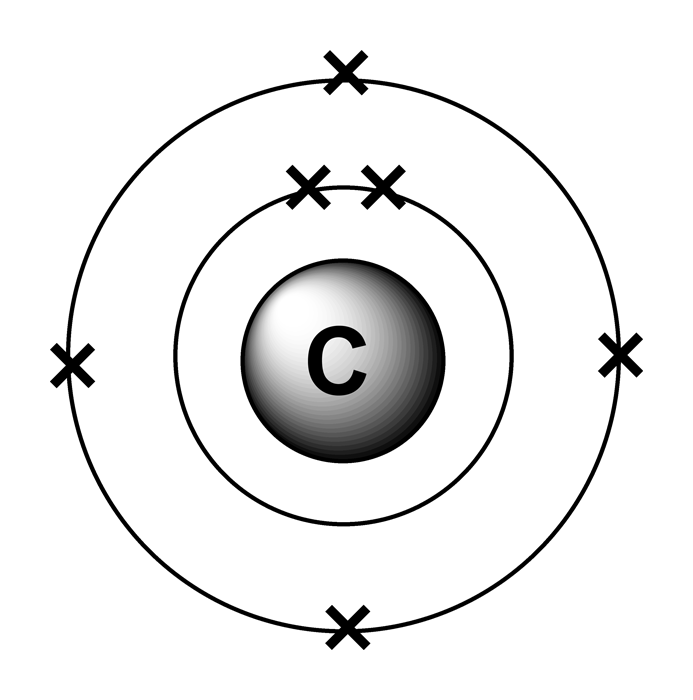
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Shell diagram of carbon (c). Atomic mass, electron configurations, charges, and more. It has six electrons in total. Carbon’s electron configuration is written as 1s² 2s² 2p². Carbon, the sixth element in the periodic table, is. View rotating bohr models for all 118. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. The electronic configuration of a carbon atom is 1s² 2s² 2p². Access detailed info on all elements:
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. View rotating bohr models for all 118. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Atomic mass, electron configurations, charges, and more. It has six electrons in total. Carbon’s electron configuration is written as 1s² 2s² 2p². Access detailed info on all elements: The electronic configuration of a carbon atom is 1s² 2s² 2p². Shell diagram of carbon (c). Carbon, the sixth element in the periodic table, is.
Carbon Electron Configuration YouTube
View rotating bohr models for all 118. Carbon, the sixth element in the periodic table, is. Shell diagram of carbon (c). Access detailed info on all elements: The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an.
Premium Vector Carbon Element 6 Electron Configuration vector
Shell diagram of carbon (c). The electronic configuration of a carbon atom is 1s² 2s² 2p². It has six electrons in total. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Atomic mass, electron configurations, charges, and more.
[Class 10 Chemistry] What is Carbon and its compounds? Teachoo
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. The electronic configuration of a carbon atom is 1s² 2s² 2p². Carbon’s electron configuration is written as 1s² 2s² 2p². Carbon, the sixth element in the periodic table, is. Access detailed info on all elements:
How Many Valence Electrons Does Carbon (C) Have?
Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118. The electronic configuration of a carbon atom is 1s² 2s² 2p². Access detailed info on all elements: It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.
Carbon(C) electron configuration and orbital diagram (2023)
Shell diagram of carbon (c). The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. The electronic configuration of a carbon atom is 1s² 2s² 2p². Access detailed info on all elements: It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.
Carbon Element With Reaction, Properties, Uses, & Price Periodic Table
Shell diagram of carbon (c). View rotating bohr models for all 118. It has six electrons in total. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. The electronic configuration of a carbon atom is 1s² 2s² 2p².
Electron configurations
The electronic configuration of a carbon atom is 1s² 2s² 2p². Access detailed info on all elements: Shell diagram of carbon (c). The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. It has six electrons in total.
Electron Configuration for Carbon (C, C4−) Full Guide
Carbon’s electron configuration is written as 1s² 2s² 2p². View rotating bohr models for all 118. Carbon, the sixth element in the periodic table, is. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Shell diagram of carbon (c).
Ce 2+ Electron Configuration
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. The electronic configuration of a carbon atom is 1s² 2s² 2p². It has six electrons in total. Atomic mass, electron configurations, charges, and more. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.
Carbon Electron Configuration Photograph by Photo
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon, the sixth element in the periodic table, is. It has six electrons in total. Shell diagram of carbon (c). Atomic mass, electron configurations, charges, and more.
Carbon’s Electron Configuration Is Written As 1S² 2S² 2P².
Shell diagram of carbon (c). The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an. Atomic mass, electron configurations, charges, and more. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.
Carbon, The Sixth Element In The Periodic Table, Is.
Access detailed info on all elements: It has six electrons in total. View rotating bohr models for all 118. The electronic configuration of a carbon atom is 1s² 2s² 2p².


![[Class 10 Chemistry] What is Carbon and its compounds? Teachoo](https://d1avenlh0i1xmr.cloudfront.net/large/c431a411-989e-4521-b718-2b9e2d1e269e/electronic-configuration-of-carbon-atom---teachoo.jpg)