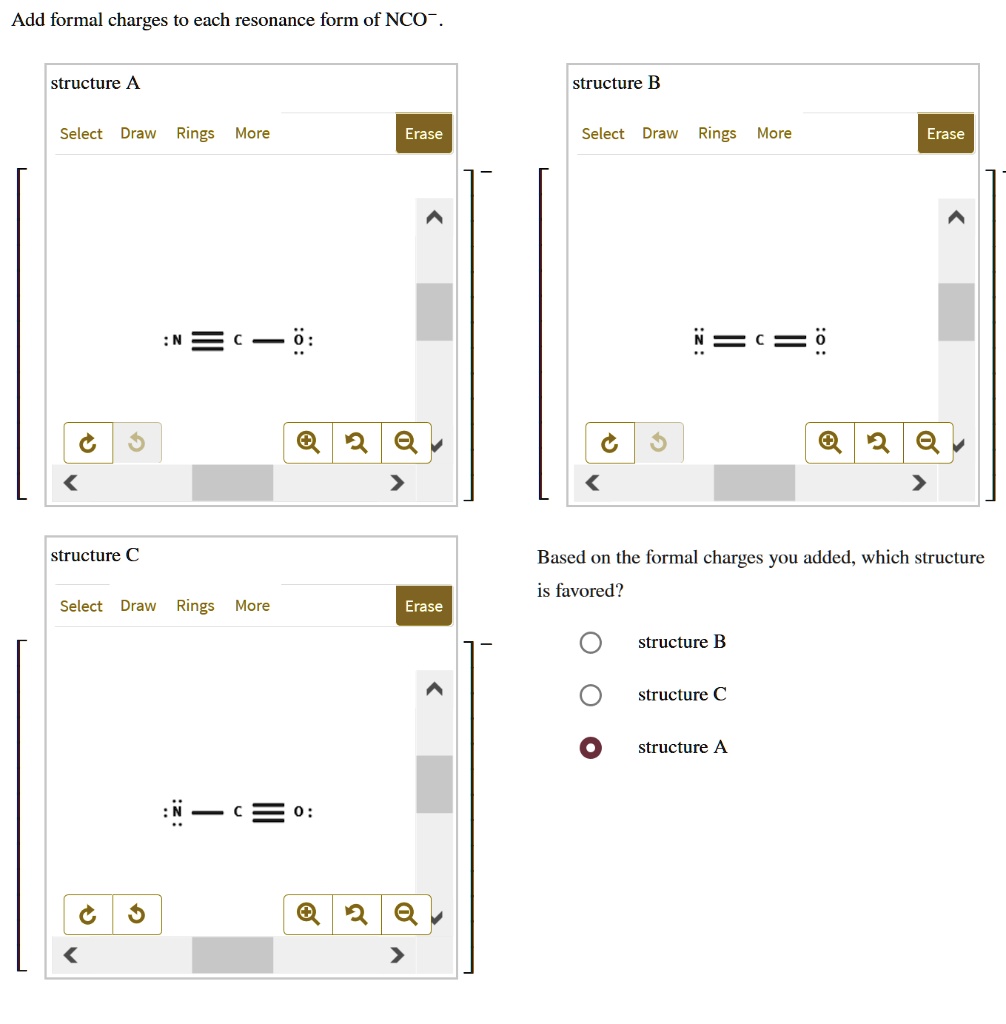
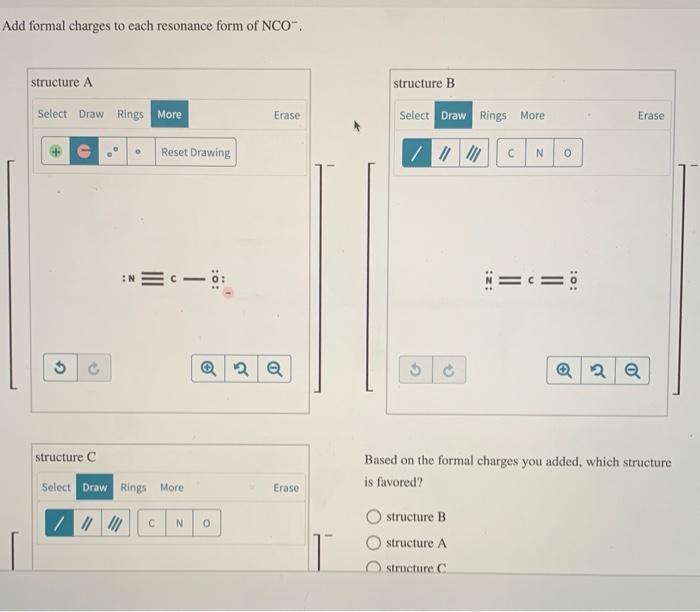
Add Formal Charges To Each Resonance Form Of Nco
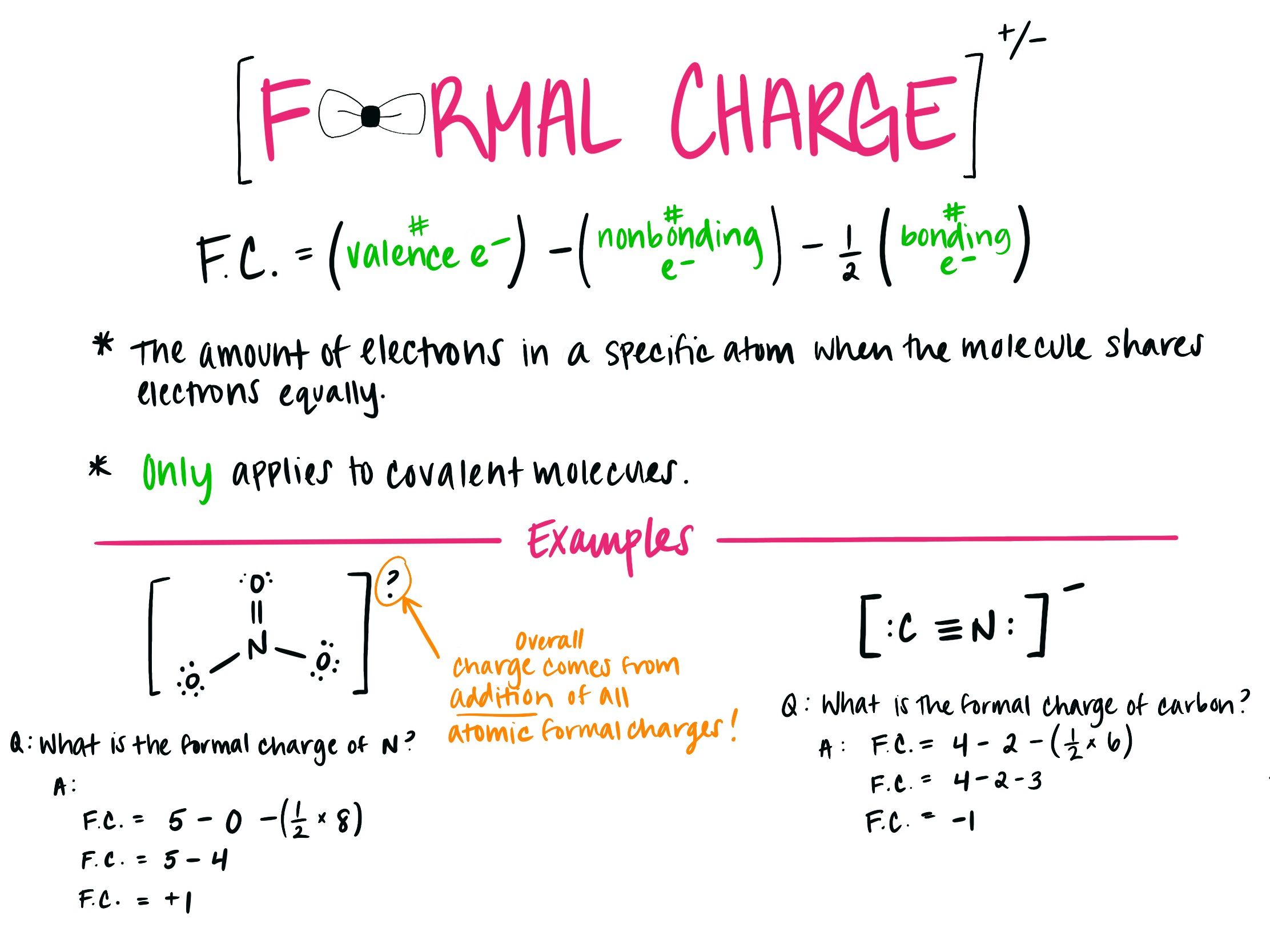
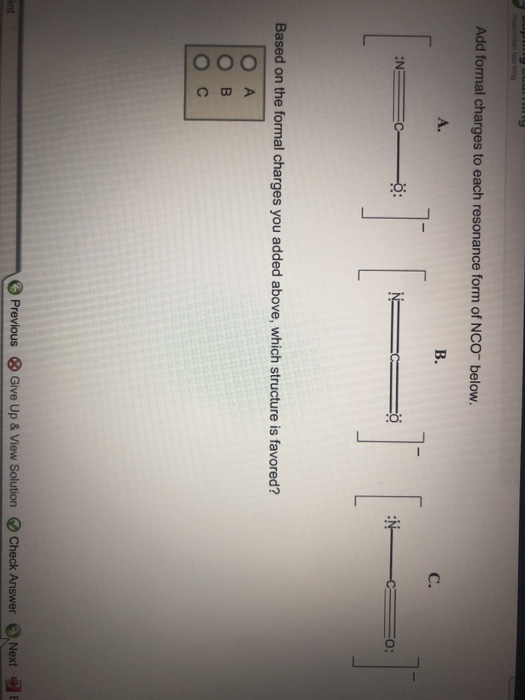
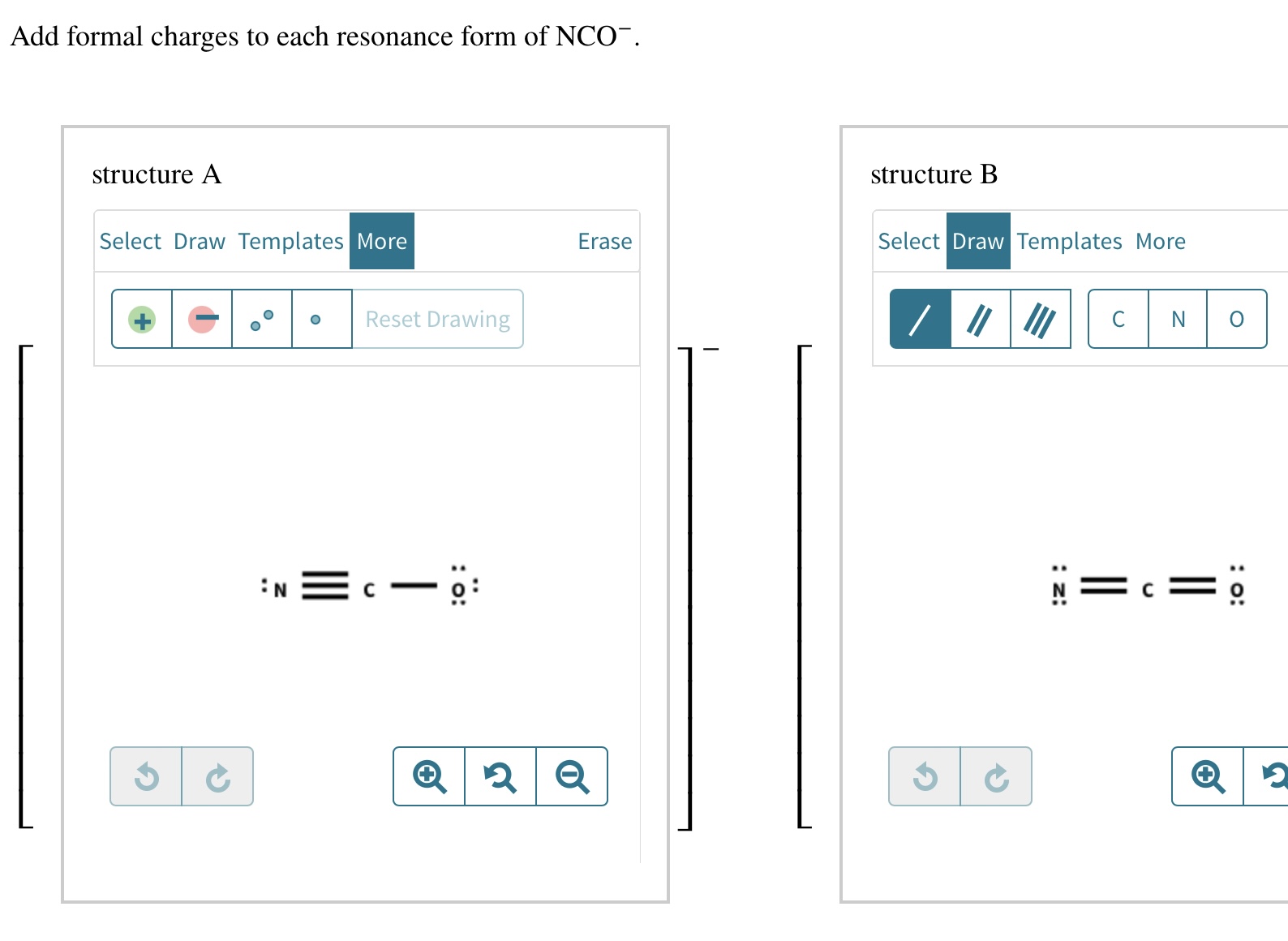
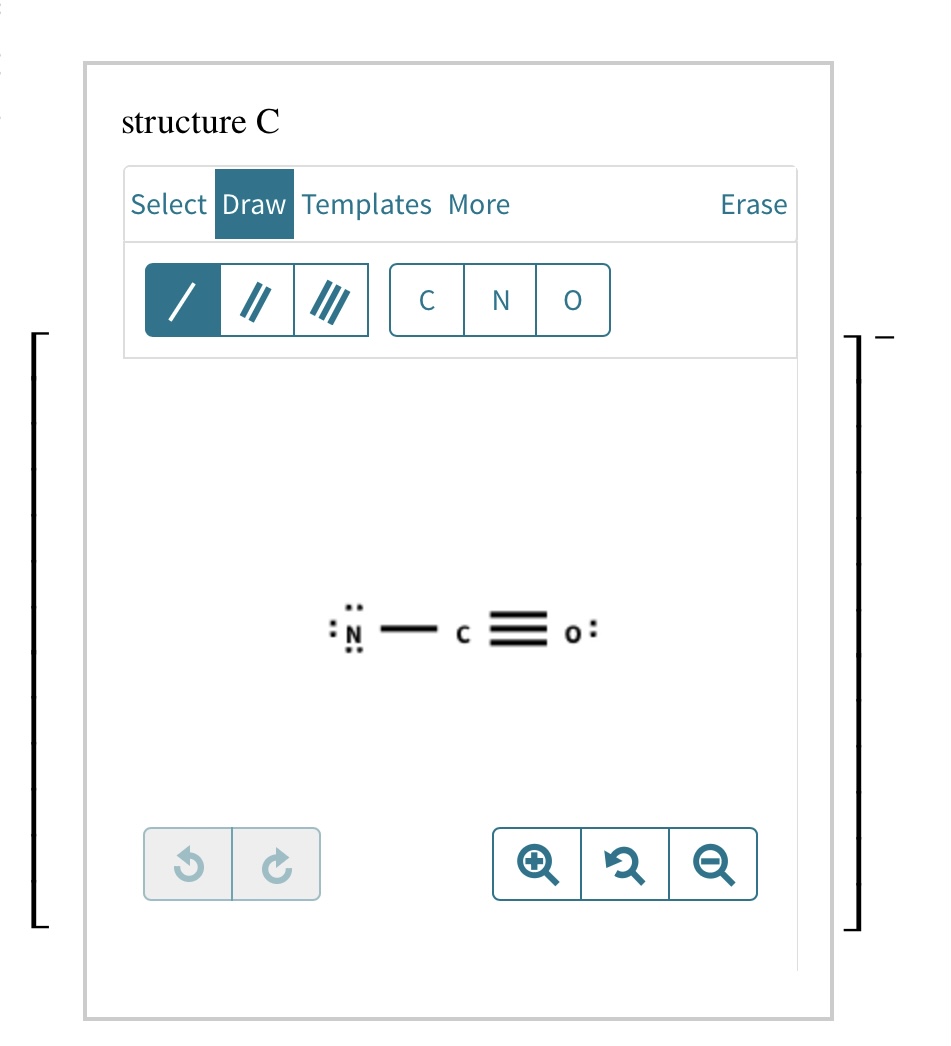
Add Formal Charges To Each Resonance Form Of Nco - When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero. Given to add formal charge to each resonance form of nco a −. Based on the formal charge need to determine th.
Given to add formal charge to each resonance form of nco a −. Based on the formal charge need to determine th. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero.
Based on the formal charge need to determine th. Given to add formal charge to each resonance form of nco a −. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero.
Chemistry Archive January 26, 2015
Based on the formal charge need to determine th. Given to add formal charge to each resonance form of nco a −. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero.
add formal charges to each resonance form of HCNO below WizEdu
Based on the formal charge need to determine th. Given to add formal charge to each resonance form of nco a −. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero.
18 Captivating Facts About Formal Charge
Based on the formal charge need to determine th. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero. Given to add formal charge to each resonance form of nco a −.
Solved y Add formal charges to each resonance form of NCO
Based on the formal charge need to determine th. Given to add formal charge to each resonance form of nco a −. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero.
Answered Add formal charges to each resonance… bartleby
When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero. Based on the formal charge need to determine th. Given to add formal charge to each resonance form of nco a −.
Solved Add formal charges to each resonance form of
Based on the formal charge need to determine th. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero. Given to add formal charge to each resonance form of nco a −.
[Solved] Add formal charges to each resonance form of NCO structure A
Given to add formal charge to each resonance form of nco a −. Based on the formal charge need to determine th. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero.
Solved Add formal charges to each resonance form of
Given to add formal charge to each resonance form of nco a −. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero. Based on the formal charge need to determine th.
SOLVED Add formal charges to each resonance form of NCO structure A
Based on the formal charge need to determine th. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero. Given to add formal charge to each resonance form of nco a −.
Solved Add formal charges to each resonance form of NCO.
Given to add formal charge to each resonance form of nco a −. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero. Based on the formal charge need to determine th.
Based On The Formal Charge Need To Determine Th.
Given to add formal charge to each resonance form of nco a −. When determining the best lewis structure or the predominant resonance structure, we want that the atoms have a formal charge close to zero.