Acids React With Bases To Form Salt And Water
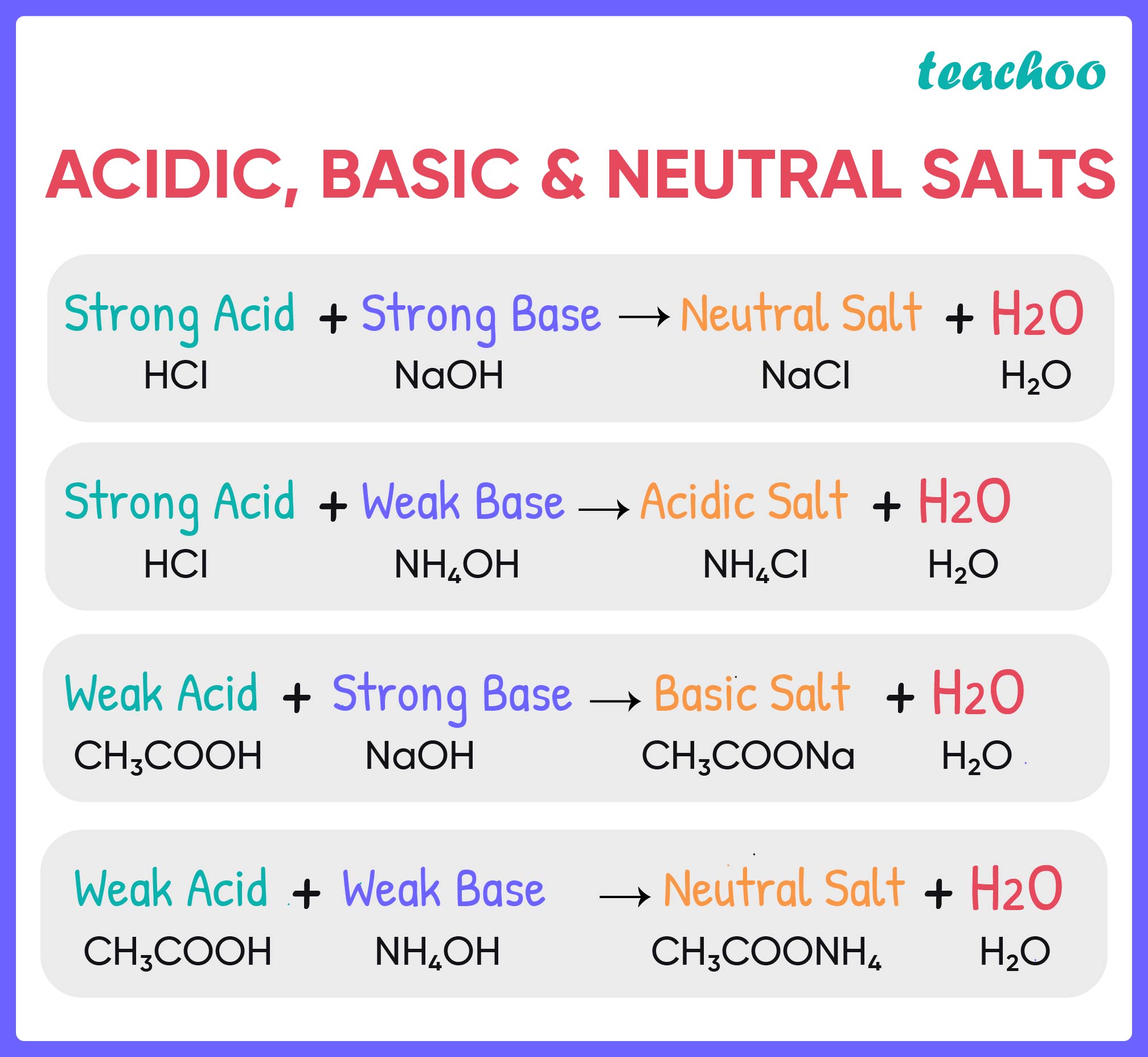
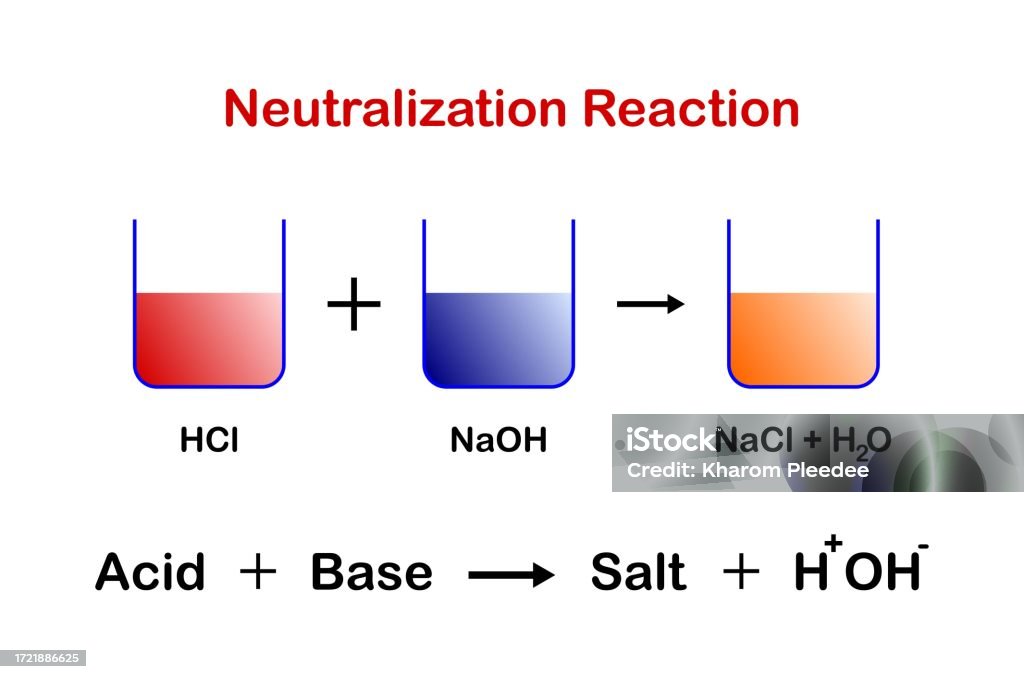
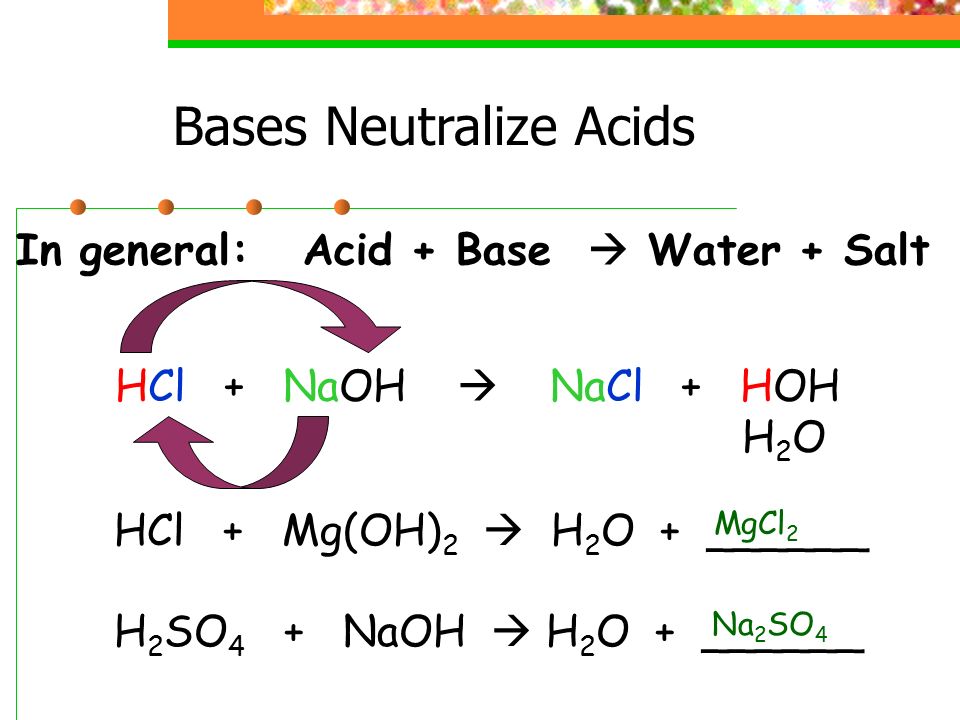
Acids React With Bases To Form Salt And Water - When an acid and a base are combined, water and a salt are the products. When an acid reacts with a base, we get salt and water as products. Hydrochloric acid reacts with sodium hydroxide to form sodium. In a neutralisation reaction, an acid and a base combine to form a. Acids react with bases to form a salt and water. Salt solutions do not always. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Salts are ionic compounds containing a positive ion other than h+ h + and. Acid + base → salt + water.
Acids react with bases in a neutralisation reaction to form salts and water. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. When an acid reacts with a base, we get salt and water as products. Hydrochloric acid reacts with sodium hydroxide to form sodium. Salts are ionic compounds containing a positive ion other than h+ h + and. Acids react with bases to form a salt and water. When an acid and a base are combined, water and a salt are the products. Salt solutions do not always. Acid + base → salt + water. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water.
Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Acids react with bases to form a salt and water. Acid + base → salt + water. Salts are ionic compounds containing a positive ion other than h+ h + and. When an acid and a base are combined, water and a salt are the products. When an acid reacts with a base, we get salt and water as products. In a neutralisation reaction, an acid and a base combine to form a. Acids react with bases in a neutralisation reaction to form salts and water. Salt solutions do not always.
Acids React With Bases To Produce Salt And Water Stock Illustration
When an acid and a base are combined, water and a salt are the products. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. In a neutralisation reaction, an acid and a base combine to form a. Hydrochloric acid reacts with sodium hydroxide to form sodium. Acid + base → salt.
A Level Chemistry Revision Physical Chemistry Acids And Bases
Hydrochloric acid reacts with sodium hydroxide to form sodium. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Salts are ionic compounds containing a positive ion other than h+ h + and. Acids react with bases in a neutralisation.
Acid Base Reaction Examples
Acids react with bases to form a salt and water. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Salts are ionic compounds containing a positive ion other than h+ h + and. In a neutralisation reaction, an acid and a base combine to form a. Salt solutions do not always.
Acids, Bases and Salts class 7 worksheet witknowlearn Acids bases
Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. When an acid and a base are combined, water and a salt are the products. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. In a neutralisation reaction, an acid and a base combine to form a. Acids react.
Lesson Plan of Properties and Uses of Acids (Acids, Alkalies and Salts
When an acid reacts with a base, we get salt and water as products. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Acids react with bases in a neutralisation reaction to form salts and water. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Hydrochloric acid reacts.
Acids react with bases to form salt and water. This reaction is known as
Salt solutions do not always. In a neutralisation reaction, an acid and a base combine to form a. Acids react with bases to form a salt and water. Acids react with bases in a neutralisation reaction to form salts and water. Salts are ionic compounds containing a positive ion other than h+ h + and.
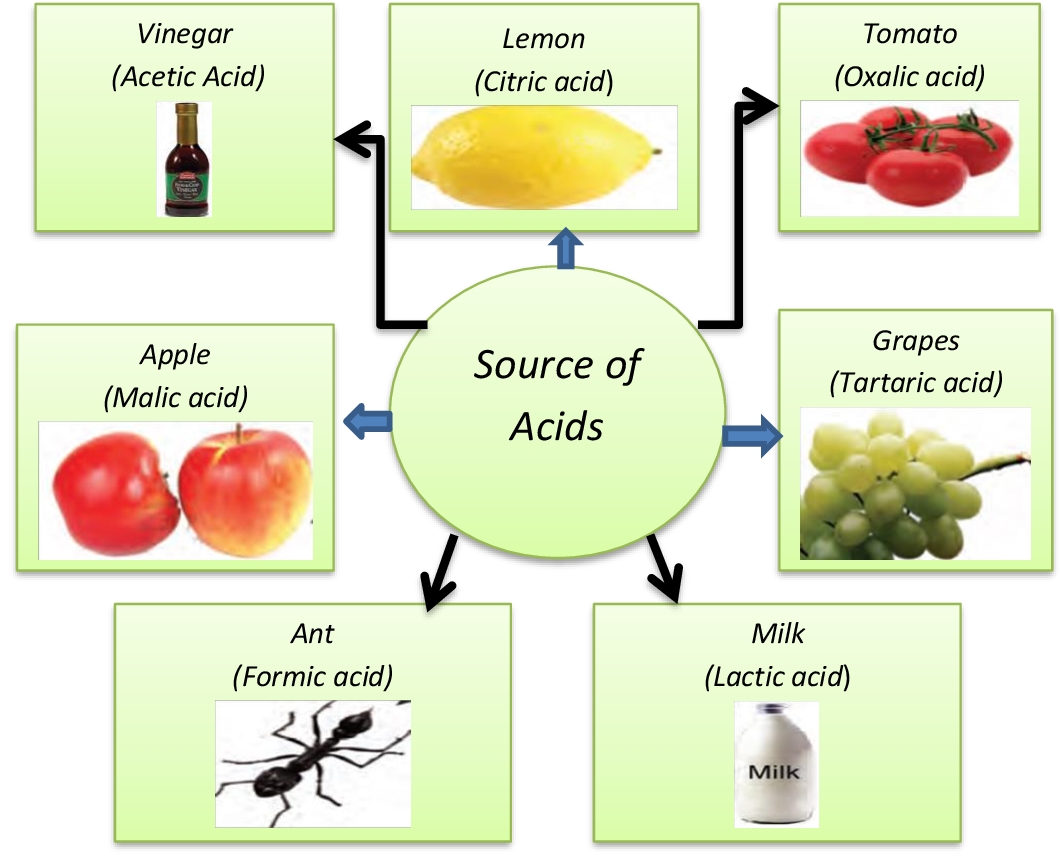
Acids and it's Properties Definition [with Flowchart and Examples]
In a neutralisation reaction, an acid and a base combine to form a. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. When an acid and a base are combined, water and a salt are the products. Acids react with bases in a neutralisation reaction to form salts and water. Acids.
Acids and Bases Science with Mrs Beggs
In a neutralisation reaction, an acid and a base combine to form a. Hydrochloric acid reacts with sodium hydroxide to form sodium. Salt solutions do not always. Acid + base → salt + water. Acids react with bases to form a salt and water.
Acids, Bases, And Salts Definition, Types, Properties, And, 51 OFF
Salt solutions do not always. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Acids react with bases in a neutralisation reaction to form salts and water. When an acid and a base are combined, water and a salt are the products. Salts are ionic compounds containing a positive ion other than h+ h + and.
Salts and it's Properties (with Examples) Acids, Bases and Salt
When an acid reacts with a base, we get salt and water as products. Hydrochloric acid reacts with sodium hydroxide to form sodium. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Acids react with bases to form a salt and water. The acid and base have neutralized each other, and the acidic and basic properties are.
Acids React With Bases To Form A Salt And Water.
Salts are ionic compounds containing a positive ion other than h+ h + and. Acid + base → salt + water. When an acid and a base are combined, water and a salt are the products. The acid and base have neutralized each other, and the acidic and basic properties are no longer present.
Salt Solutions Do Not Always.
In a neutralisation reaction, an acid and a base combine to form a. When an acid reacts with a base, we get salt and water as products. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Hydrochloric acid reacts with sodium hydroxide to form sodium.




![Acids and it's Properties Definition [with Flowchart and Examples]](https://d1avenlh0i1xmr.cloudfront.net/28568581-671c-4933-be3c-3b6bced35321/some-properties-of-acids-teachoo.jpg)
.png)